Researchers identify protein that drives the development of alcohol-associated liver disease
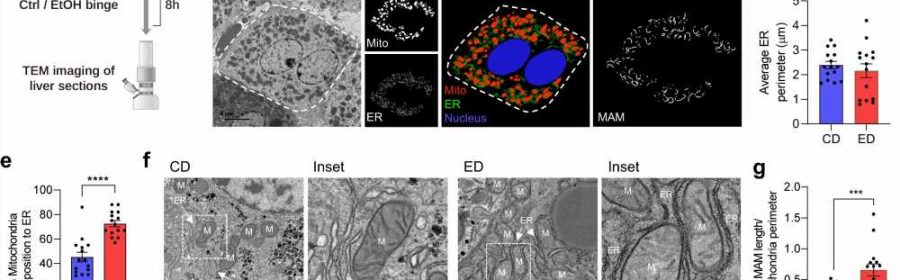
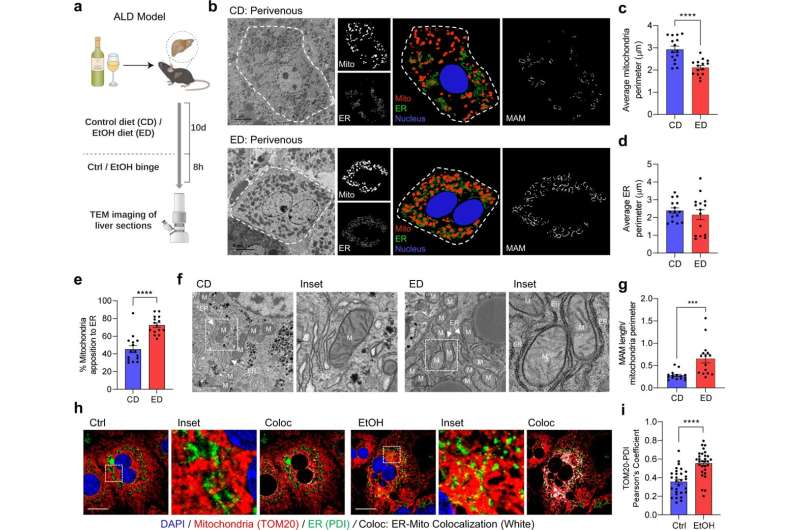
Alcohol-associated liver disease (ALD), a complex disorder that occurs in some patients who have engaged in excessive alcohol use, is one of the leading causes of chronic liver disease among veterans and liver transplant patients in the United States. Despite its prevalence, and despite many recent advances in the hepatology field, no new treatment interventions for ALD have been developed over the past several decades.
This may change based on a recent discovery by a team led by Suthat Liangpunsakul, MD, MPH, of Indiana University School of Medicine. Liangpunsakul’s team have identified the novel role of a protein called “Pyruvate dehydrogenase kinase 4,” or PDK4, in the development of ALD, which may pave the way for new treatments.
The team published their findings in the paper “Enhanced Ca2+-channeling complex formation at the ER-mitochondria interface underlies the pathogenesis of alcohol-associated liver disease” in Nature Communications in March.
In the paper, Liangpunsakul and his team report that PDK4 plays a mediatory role with the formation of the membrane of the mitochondria-associated endoplasmic reticulum, which drives calcium accumulation in the mitochrondria.
PDK4 is therefore a key mediator of alcohol-induced liver injury, said Liangpunsakul, who is a professor of medicine in the school’s Division of Gastroenterology and Hepatology and staff physician at the Richard L. Roudebush Veterans’ Administration Medical Center.
“The accumulation of calcium in the mitochondria has been linked to mitochondrial dysfunction. However, the factors involved in promoting mitochondrial calcium accumulation and dysfunction during the pathogenesis of ALD have not been well understood” until now, he said.
Robert A. Harris, Ph.D., who is a distinguished professor emeritus and former chair of the Department of Biochemistry and Molecular Biology at IU School of Medicine, is also an author of the study. He noted that we already know that PDK4, an essential protein in glucose homeostasis, plays an important role in metabolic conditions such as diabetes and cancer.
“This novel study extends the role of PDK4 on alcohol-associated liver disease, notably on its effect on mitochondria and endoplasmic reticulum,” he said, emphasizing the importance of PDK4 as a “potential therapeutic target” for ALD.
Investigators from Kyungpook National University, Kyungpook National University Hospital, Daegu, Republic of Korea, were also involved in the study. It was partly supported by the National Institutes of Health and the National Institute on Alcohol Abuse and Alcoholism; a VA Merit Award; and IU School of Medicine.
More information:
Themis Thoudam et al, Enhanced Ca2+-channeling complex formation at the ER-mitochondria interface underlies the pathogenesis of alcohol-associated liver disease, Nature Communications (2023). DOI: 10.1038/s41467-023-37214-4
Journal information:
Nature Communications
Source: Read Full Article