Antibodies against SARS-CoV-2 are higher in infants than in mothers

In a recent study posted to the bioRxiv* preprint server, researchers characterize the functional and binding antibody responses against the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in plasma samples obtained from postpartum women and their infected infants from an unvaccinated population in Nairobi, Kenya.

Study: Elevated binding and functional antibody responses to SARS-CoV-2 in infants versus mothers. Image Credit: Herlanzer / Shutterstock.com
Background
Several studies have reported that, due to differences in the immune systems of infants and adults, as well as variations in exposure to pathogens, antibody responses during viral infection in infants are different from that in adults. While research on the protection elicited by vaccines and previous SARS-CoV-2 infections indicate that the levels of plasma antibodies targeting the viral spike protein are correlates of protection, there remains a lack of information on the antibody responses to SARS-CoV-2 in infants.
Several antibody epitopes for SARS-CoV-2 have been identified, including those within the receptor binding domain (RBD) of the S1 subunit of the viral spike protein, as well as the fusion machinery housed in the S2 subunit.
While the RBD is the target of most neutralizing antibodies, plasma antibodies may also bind to non-RBD regions of the spike protein, such as the S2 subunit regions. These regions are potential therapeutic targets, as they do appear to be under selection for new mutations. However, whether these plasma antibody epitopes are also prominent in infants, as well as the extent to which the antibody profiles of infants differ from those of their mothers, is not known.
About the study
In the present study, plasma samples were collected from mother-infant pairs enrolled in a prospective cohort study in Nairobi, Kenya. The participants were tested for SARS-CoV-2 seropositivity using the nucleocapsid enzyme-linked immunosorbent assay (ELISA).
Seropositive mothers had seroconverted after giving birth, thus ensuring that the anti-SARS-CoV-2 antibodies found in the infant were not a result of passive transfer from mother to fetus. Furthermore, previous studies have shown that antibodies in breast milk do not circulate in infants in appreciable amounts.
The study cohort consisted of mothers who were positive or negative for human immunodeficiency virus (HIV) and infants who were exposed and uninfected or unexposed to HIV. Nevertheless, HIV status did not appear to influence SARS-CoV-2 infection risk.
While none of the participants had received coronavirus disease 2019 (COVID-19) vaccines, the SARS-CoV-2 infections in all participants were asymptomatic or mild.
The first seropositive plasma samples of the mother and infant were compared using a multiplexed electrochemiluminescence platform (MSD) and a cell-surface staining assay. The MSD approach detects immunoglobulin G (IgG) antibodies binding to the SARS-CoV-2 spike protein, RBD, N-terminal domain (NTD), and nucleocapsid protein, while the cell-surface staining assay detects antibodies binding to a green fluorescent protein-tagged spike protein.
A phage-based immunoprecipitation method was used to differentiate the binding sites and identify antibody epitopes in the infants and mothers. This approach also defined mutations in the binding sites by evaluating the loss in antibody binding due to mutated peptides.
Antibodies eBook

The scaled differential selection metric was used to assess the impact of a mutation on antibody binding. Escape similarity scores were calculated to evaluate whether escape profiles were present to a greater degree in mothers or infants.
Higher SARS-CoV-2 antibody levels in infants
Infants had significantly higher antibody titers against the SARS-CoV-2 spike protein, RBD, and NTD. Furthermore, surface staining of cells expressing spike proteins was also higher for infants.
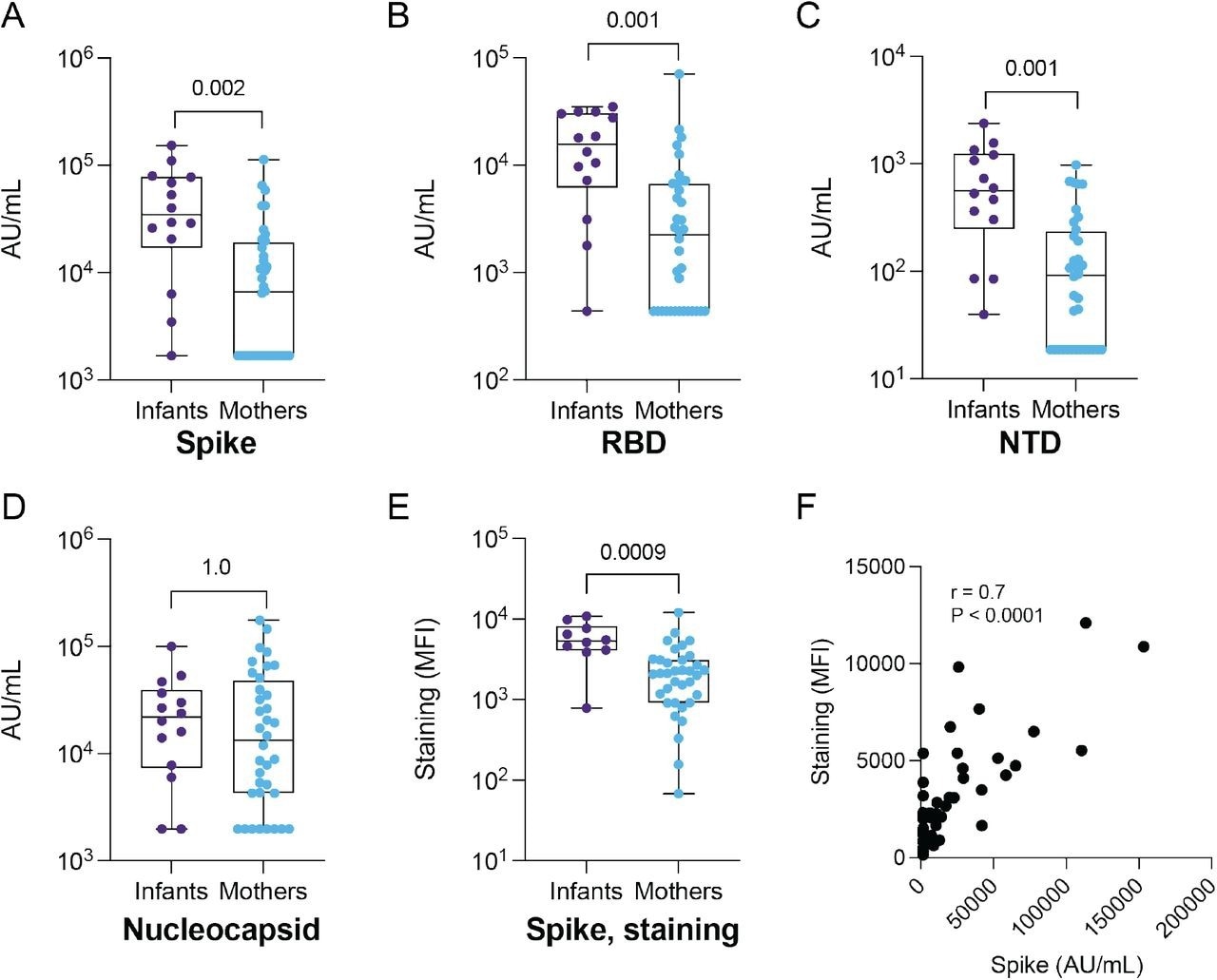
IgG binding to SARS-CoV-2 antigens and S-CEM cell surface staining in SARS-CoV-2-seropositive infants and mothers. IgG antibody binding titers to (A) full-length Spike, (B) RBD, (C) NTD and (D) nucleocapsid in convalescent plasma from infants (N = 14) and mothers (N = 35) measured by commercial multiplexed electrochemiluminescent assay (MSD). (E) Antibody binding to Spike expressed on the CEM cell surface in infants (N = 10) and mothers (N = 35). The Spike staining was repeated in triplicate. (F) Spearman correlation coefficient and p-value calculated for Spike IgG binding by MSD assay versus S-CEM cell surface staining. P-values are indicated above comparisons. Two-tailed Wilcoxon rank sum test was used for all comparisons. MFI: mean fluorescence intensity.
Plasma antibodies of both mothers and infants bound the same epitopes in the spike S2 subunit, such as the stem helix-heptad repeat 2 and fusion peptide. However, in the infants, these antibody levels were higher and displayed greater antibody escape pathways in the fusion peptide region than those in the mothers.
Furthermore, while neutralizing antibody titers were similar for mothers and infants, infants displayed higher levels of antibody-dependent cellular cytotoxicity, which has been linked to protection against viruses such as HIV and SARS-CoV-2. These findings suggest that the functional antibody repertoires developed in infants during SARS-CoV-2 infection were unique.
The fusion peptide antibody escape pathways were similar between infants, despite being different for infant-mother pairs. This suggests that antibody escape pathways might be a factor of age, rather than genetics.
Conclusions
The functional and binding plasma antibodies against SARS-CoV-2, as well as antibody escape pathways, were found to differ between SARS-CoV-2-infected infants and mothers, with infants exhibiting higher levels of antibody-dependent cellular cytotoxicity.
These findings suggest distinct antibody repertoires present in infants, with age-related differences in immune responses to SARS-CoV-2. Moreover, these observations provide important information for the design and evaluation of future COVID-19 vaccines and therapeutics for infants.
*Important notice
bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
- Stoddard, C. I., Sung, K., Yaffe, Z. A., et al. (2023). Elevated binding and functional antibody responses to SARS-CoV-2 in infants versus mothers. bioRxiv. doi:10.1101/2023.02.06.527330. https://www.biorxiv.org/content/10.1101/2023.02.06.527330v1
Posted in: Child Health News | Medical Science News | Medical Research News | Women's Health News | Disease/Infection News
Tags: Antibodies, Antibody, Assay, Breast Milk, Cell, Convalescent Plasma, Coronavirus, Coronavirus Disease COVID-19, covid-19, Cytotoxicity, ELISA, Enzyme, Fluorescence, Fluorescent Protein, Genetics, Helix, HIV, Immunodeficiency, Immunoglobulin, Immunoprecipitation, Mutation, Peptides, Protein, Receptor, Research, Respiratory, SARS, SARS-CoV-2, Severe Acute Respiratory, Severe Acute Respiratory Syndrome, Spike Protein, Syndrome, Therapeutics, Virus
.jpg)
Written by
Dr. Chinta Sidharthan
Chinta Sidharthan is a writer based in Bangalore, India. Her academic background is in evolutionary biology and genetics, and she has extensive experience in scientific research, teaching, science writing, and herpetology. Chinta holds a Ph.D. in evolutionary biology from the Indian Institute of Science and is passionate about science education, writing, animals, wildlife, and conservation. For her doctoral research, she explored the origins and diversification of blindsnakes in India, as a part of which she did extensive fieldwork in the jungles of southern India. She has received the Canadian Governor General’s bronze medal and Bangalore University gold medal for academic excellence and published her research in high-impact journals.
Source: Read Full Article