AD Outcomes Improved With Lebrikizumab and Topical Steroids
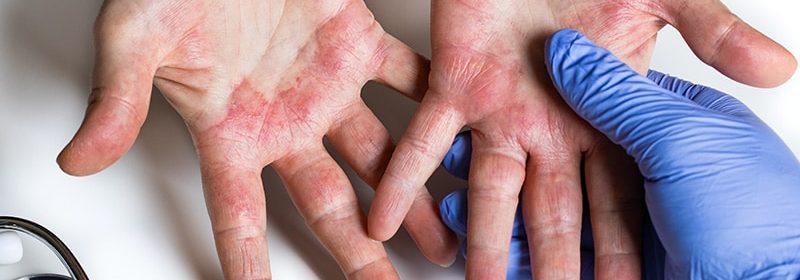
Adult and adolescent patients with moderate to severe atopic dermatitis (AD) showed significant improvements with the addition of lebrikizumab to topical steroid therapy (TCS), compared with TCS plus placebo, according to results of the 16-week phase 3 ADhere trial.
“Lebrikizumab, a monoclonal antibody inhibiting interleukin-13, combined with TCS was associated with reduced overall disease severity of moderate-to-severe AD in adolescents and adults, and had a safety profile consistent with previous lebrikizumab AD studies,” noted lead author Eric L. Simpson, MD, professor of dermatology, Oregon Health & Science University, Portland, and coauthors in their article on the study, which was published on January 11 in JAMA Dermatology.
The double-blind trial, conducted at 54 sites across Germany, Poland, Canada, and the United States, included 211 patients, mean age 37.2 years, of whom 48.8% were female and roughly 22% were adolescents. Almost 15% were Asian persons, and about 13% were Black persons.
At baseline, participants had a score of 16 or higher on the Eczema Area and Severity Index (EASI), a score of 3 or higher on the Investigator’s Global Assessment (IGA) scale, AD covering a body surface area of 10% or greater, and a history of inadequate response to treatment with topical medications.
After a minimum 1-week washout period from topical and systemic therapy, participants were randomized in a 2:1 ratio to receive lebrikizumab plus TCS (n = 145) or placebo plus TCS (n = 66) for 16 weeks.
Lebrikizumab or placebo was administered by subcutaneous injection every 2 weeks; the loading and week 2 doses of lebrikizumab were 500 mg, followed by 250 mg thereafter. All patients were instructed to use low- to mid-potency TCS at their own discretion. Study sites provided a mid-potency TCS (triamcinolone acetonide 0.1% cream) and a low-potency TCS (hydrocortisone 1% cream), with topical calcineurin inhibitors permitted for sensitive skin areas.
Primary outcomes at 16 weeks included a 2-point or more reduction in IGA score from baseline and EASI-75 response. Patients in the lebrikizumab arm had superior responses on both of these outcomes, with statistical significance achieved as early as week 8 and week 4, respectively, and maintained through week 16. Specifically, 41.2% of those treated with lebrikizumab had an IGA reduction of 2 points or more, compared with 22.1% of those receiving placebo plus TCS (P = .01), and the proportion of patients achieving EASI-75 responses was 69.5% vs 42.2%, respectively (P < .001).
Patients treated with lebrikizumab also showed statistically significant improvements compared with TCS alone in all key secondary endpoints, “including skin clearance, improvement in itch, itch interference on sleep, and enhanced QoL [quality of life],” noted the authors. “This study captured the clinical benefit of lebrikizumab through the combined end point of physician-assessed clinical sign of skin clearance (EASI-75) and patient-reported outcome of improvement in itch (Pruritus NRS).”
The percentage of patients who achieved the combined endpoint was more than double for the lebrikizumab plus TCS group vs the group on TCS alone, indicating that patients treated with lebrikizumab plus TCS “were more likely to experience improvement in skin symptoms and itch,” the investigators added.
The authors noted that most treatment-emergent adverse events “were nonserious, mild or moderate in severity, and did not lead to study discontinuation.” These included conjunctivitis (4.8%), headache (4.8%), hypertension (2.8%), injection-site reactions (2.8%), and herpes infection (3.4%) — all of which occurred in 1.5% or less of patients in the placebo group.
“The higher incidence of conjunctivitis has also been reported in other biologics inhibiting IL-13 and/or IL-4 signaling, as well as lebrikizumab monotherapy studies,” they noted. The 4.8% rate of conjunctivitis reported in the combination study, they added, is “compared with 7.5% frequency in 16-week data from the lebrikizumab monotherapy studies. Although the mechanism remains unclear, it has been reported that conjunctival goblet cell scarcity due to IL-13 and IL-4 inhibition, and subsequent effects on the homeostasis of the conjunctival mucosal surface, results in ocular AEs [adverse events].”
“This truly is a time of great hope and promise for our patients with AD,” commented Zelma Chiesa Fuxench, MD, who was not involved in the study. “The advent of newer, targeted therapeutic agents for AD continues to revolutionize the treatment experience for our patients, offering the possibility of greater AD disease control with a favorable risk profile and less need for blood work monitoring compared to traditional systemic agents.”
On the basis of the study results, Chiesa Fuxench, of the Department of Dermatology at the University of Pennsylvania, Philadelphia, said in an interview that “lebrikizumab represents an additional option in the treatment armamentarium for providers who care for patients with AD.” She added that “while head-to-head trials comparing lebrikizumab to dupilumab, the first FDA-approved biologic for AD, would be beneficial, to the best of my knowledge this data is currently lacking. However, based on the results of this study, we would expect lebrikizumab to work at least similarly to dupilumab based on the reported improvements in IGA and EASI score.”
Additionally, she said lebrikizumab showed a favorable safety profile, “with most treatment-emergent adverse effects reported as nonserious and not leading to drug discontinuation,” she said. “Of interest to clinicians may be the reported rates of conjunctivitis in this study. Rates of conjunctivitis for lebrikizumab appear to be lower than those reported in the LIBERTY AD CHRONOS study for dupilumab — a finding that merits further scrutiny in my opinion, as this one of the most frequent treatment-emergent adverse events that I encounter in my clinical practice.”
The study was funded by Dermira, a subsidiary of Eli Lilly. Simpson reported personal fees and grants from multiple sources, including Dermira and Eli Lilly, the companies developing lebrikizumab. Coauthor disclosures included reporting grants, advisory roles, and speaker and/or personal fees from Eli Lilly, and Dermira; nonfinancial support from Eli Lilly; and/or serving as a speaker/receiving honoraria from and acting as a clinical study investigator/institution receiving clinical study funds from Eli Lilly. Several authors were employees of Eli Lilly. Chiesa Fuxench disclosed serving as a consultant for the Asthma and Allergy Foundation of America, National Eczema Association, Pfizer, AbbVie, and Incyte, for which she has received honoraria for AD-related work. She is the recipient of research grants through Regeneron, Sanofi, Tioga, Vanda, Menlo Therapeutics, Leo Pharma, and Eli Lilly for work related to AD as well as honoraria for continuing medical education work related to AD sponsored through educational grants from Regeneron/Sanofi and Pfizer.
JAMA Dermatol. Published online January 11, 2023. Full text
Kate Johnson is a Montreal-based freelance medical journalist who has been writing for more than 30 years about all areas of medicine.
For more news, follow Medscape on Facebook, Twitter, Instagram, and YouTube
Source: Read Full Article