Why do Vaccines need to be Refrigerated?

Vaccines must be stored in specific conditions to avoid degradation, usually defined specifically by the manufacturer. Across the stages of manufacture, distribution, storage, and ultimately administration, these conditions must be adhered to, and this process is known as the cold chain.
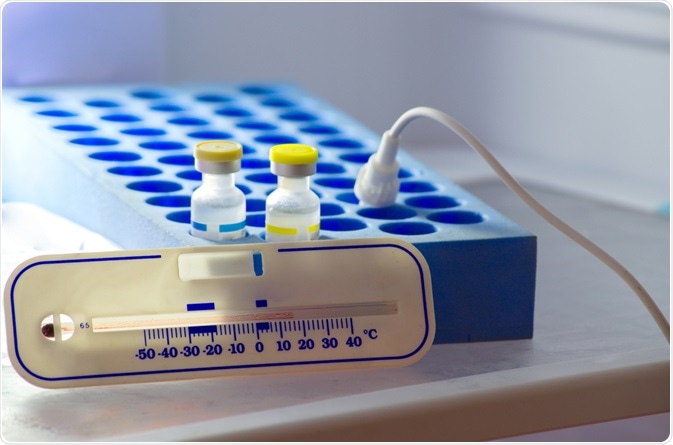
Image Credit: Anukool Manoton/Shutterstock.com
Should the cold chain be broken at any point during transport or storage, via exposure to temperate extremes, then the potency of the vaccine risks being reduced, or the vaccine is even rendered completely ineffective.
The vast majority of vaccines must be refrigerated at between 2-8⁰C, with a preferred average of 5⁰C with minimal fluctuations. Specially designed lab refrigerators are usually used for this purpose, which has comparatively minimal temperature fluctuation across days and seasons, do not present any temperature extremes on any interior surface, and may bear an external temperature display that automatically logs the internal temperature at particular time intervals.
Many live vaccines tolerate freezing, and depending on the specific manufacturer’s instructions are instead frozen at between -15 and -50⁰C. Among commonly administered vaccines this includes just varicella (chickenpox), zoster (shingles), and smallpox, with most others instead of being refrigerated.
Most non-replicating vaccines: inactivated viruses or bacteria, purified protein subunits, carbohydrate antigens, and recombinant subunit protein antigens, are administered alongside adjuvants such as aluminum salts. Aluminum salts have been used in vaccines around the world for nearly a century, acting to form an ionic bond with the antigen of interest in the vaccine, massively improving stability and potency.
In more recent years additional potentially extremely significant purpose has been found in the use of aluminum salt adjuvants, as they appear to aid in promoting an enhanced host immune response following administration alongside a vaccine. Aluminum salts act on monocytes, macrophages, and granulocytes to induce cytokines, generating a local immunostimulatory environment. They may also induce local necrosis of stromal cells, causing the release of uric acid that then activates inflammasomes.
In any case, aluminum salts are highly sensitive to damage by freezing, as freeze-thaw cycles cause aggregation and sedimentation of the colloidal particles. High temperatures cause almost no effect on the structure of the aluminum gel.
Indeed, freeze damage is often far more impactful than heat-related damage for vaccines, though most manufacturers recommend not allowing them to sit at room temperature for more than thirty minutes except for in some special cases. At extreme temperatures approaching and above 45⁰C the proteins present in the vaccine become denatured relatively quickly, eventually completely losing potency as the structure of the antigen is no longer present.
Kumar et al. (1982) found that a tetanus vaccine could survive at temperatures of 35⁰C for several weeks, while at 45⁰C they experienced a 5% loss in potency per day for the first two weeks of storage. When exposed to temperatures of 60⁰C the vaccine was rendered completely ineffective after three to five hours. Conversely, when stored at -30⁰C for twelve hours a tetanus vaccine lost around 30% potency.
The proteins present within the vaccine can be directly damaged by freeze-thaw cycles by several mechanisms. During fast freezing small ice crystals are formed, which necessarily present a larger surface area to the proteins and thus are more likely to come into contact, causing damage and partial unfolding.
Larger ice crystals cause more drastic damage, engulfing the proteins and potentially damaging the vaccine container. When thawing, the recrystallization process exerts tension and shear stress on the proteins.
Storing vaccines at cool temperatures also lessens the need for other preservatives and lessens the risk of bacterial growth within the vaccine. Various other chemicals may be present in a vaccine, such as traces of antibiotics from the manufacturing process, stabilizers such as sorbitol, and acidity regulators such as histidine, all of which may in turn be affected by extreme temperatures.
Sources:
- www.paho.org/…/Chapter5-Vaccine-Storage-and-Handling.pdf?ua=1
- https://www.sciencedirect.com/science/article/pii/S1045105614000487
- https://www.sciencedirect.com/science/article/pii/B9780323357616000663
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4313107/
- www.researchgate.net/…/23439250_Characterization_of_the_freeze_sensitivity_of_a_hepatitis_B_vaccine
- pubmed.ncbi.nlm.nih.gov/…/
Further Reading
- All Vaccine Content
- What are Vaccines?
- Vaccine History
- Vaccine Schedule
- Vaccine Effectiveness
Last Updated: Nov 11, 2020

Written by
Michael Greenwood
Michael graduated from Manchester Metropolitan University with a B.Sc. in Chemistry in 2014, where he majored in organic, inorganic, physical and analytical chemistry. He is currently completing a Ph.D. on the design and production of gold nanoparticles able to act as multimodal anticancer agents, being both drug delivery platforms and radiation dose enhancers.
Source: Read Full Article