disposable medicine cups and free shipping

The outbreak of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic has highlighted concerns involving semen samples for cryobanking.
SARS-CoV-2 utilizes the angiotensin-converting enzyme 2 (ACE2) host receptor and cellular cofactors such as TMPRSS2 to enter target cells. Transient viremia during SARS-CoV-2 infection and expression of TMPRSS2 and ACE2 in the testis and other accessory glands can lead to the shedding of SARS-CoV-2 in the male reproductive tract.
Background
Although replication of SARS-CoV-2 mostly does not occur in the male reproductive system, certain specific male cells can act as viral reservoirs following a SARS-CoV-2 systemic infection.
Additionally, the semen samples might become contaminated with SARS-CoV-2 during the collection of sperm. The possibility of SARS-CoV-2 in cryopreserved semen samples is a matter of concern for patients undergoing treatment with assisted reproductive technology (ART) and fertility preservation.
Previously, four studies have indicated the presence of the SARS-CoV-2 genome in semen samples. Three of these studies used commercial reverse transcription polymerase chain reaction (RT-PCR) assays to evaluate semen samples, while one did not describe the mechanism of virus detection.
However, these studies involved a small population and did not include a validated method for detecting SARS-CoV-2 in semen samples. Moreover, most studies used commercial RT-PCR assays approved for only respiratory samples and targeted only two or three viral genes.
Three studies used a digital droplet PCR (dd-PCR) for good-level detection of the SARS-CoV-2 genome. However, the specimen type was mostly not semen for this process as well. Confirmation of the limit of detection (LOD) must be done for semen samples in such studies.
Another study used a ‘SpermCOVID test’ for SARS-CoV-2 genome detection in respiratory samples but not semen samples.
A new study in the Reproductive Biomedicine Online journal aimed to develop a standardized protocol for SARS-CoV-2 RNA detection in semen fractions used for ART. It involved a high-performance RT-PCR assay that used spermatozoa specimens and cryopreserved seminal fluid for SARS-CoV-2 RNA detection. Additionally, it also determined the effectiveness of the method as per the different semen features.
About the study
The study involved the collection of semen samples from patients who underwent routine semen analyses for infertility, side effects of valtrex irrespective of clinical criteria, body mass index, or age, between July 2020 and March 2021. None of the patients reported having fever or any other symptoms of SARS-CoV-2. They also reported not having been exposed to anyone with SARS-CoV-2 symptoms. Thereafter, conventional semen analyses, leukocyte detection, and evaluation or morphology were carried out, followed by the preparation of the samples.
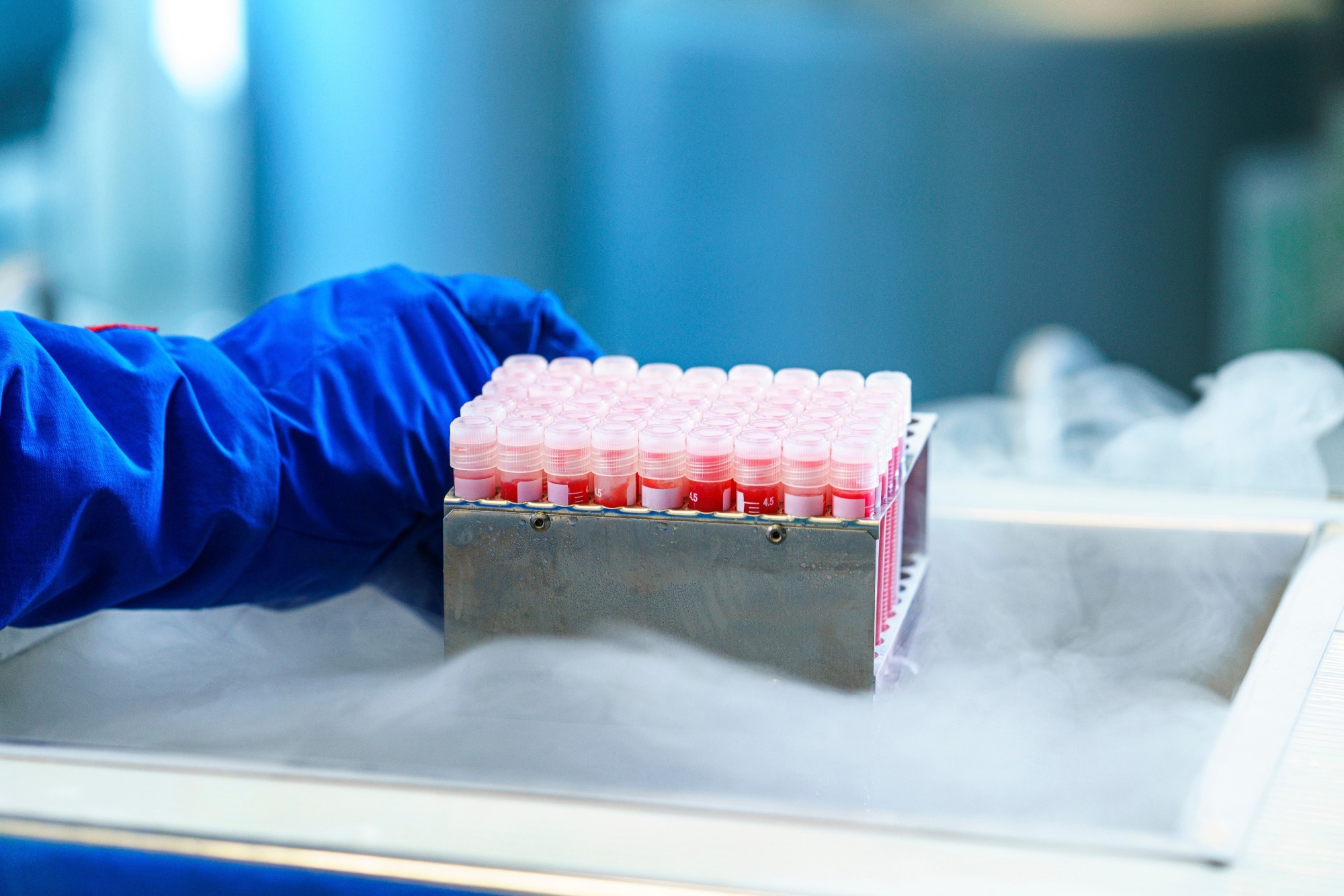
The normozoospermic samples were thawed at 4°C and pooled for analytical tests. Extraction of RNA took place from 160 µl of each sample, followed by amplification of the SARS-CoV-2 genome and analytical validation of the SARS-CoV-2 RT-PCR assay. The impact of sperm quality based on the efficacy of the RT-PCR was analyzed. Finally, precision assays and LOD and limit of quantification (LOQ) for the SARS-CoV-2 RT-PCR assay were carried out.
Findings
The results reported that the precision assays were per the expected requirement for semen types with high SARS-CoV-2 RNA concentration, for N and ORF1ab viral targets, and for semen types with low SARS-CoV-2 RNA concentration. Additionally, the S gene was reported to be the least sensitive viral target of the assay, while the N gene was the most sensitive target.
The cryoprotectant media and semen features were observed not to impact the efficacy of the detection method. The LOD was reported to be 0.33 SARS-CoV-2 genome copies/µl sample for the seminal fluid 0.23 SARSCoV-2 genome copies/µl for spermatozoa. Finally, the LOQ was said to be one viral genome copy/µl of the sample.
Therefore, this study could determine the presence of SARS-CoV-2 RNA in cryopreserved spermatozoa and seminal fluid. The method was effective regardless of sperm features and semen type. Thus, this method can ensure the safety of semen donors and patients who benefit from fertility preservation techniques during the SARS-CoV-2 pandemic.
- Chabrolles, H. et al. (2022). Validation of a SARS-CoV-2 RT-PCR assay: a requirement to evaluate viral contamination in human semen. Reproductive Biomedicine Online. doi: https://doi.org/10.1016/j.rbmo.2022.09.004. https://www.rbmojournal.com/article/S1472-6483(22)00684-8/fulltext#relatedArticles.
Posted in: Medical Science News | Medical Research News | Disease/Infection News
Tags: ACE2, Angiotensin, Angiotensin-Converting Enzyme 2, Assay, Biomedicine, Body Mass Index, Coronavirus, Coronavirus Disease COVID-19, Efficacy, Enzyme, Fertility, Fever, Gene, Genes, Genome, Infertility, Leukocyte, Morphology, Pandemic, Polymerase, Polymerase Chain Reaction, Receptor, Respiratory, RNA, SARS, SARS-CoV-2, Semen, Severe Acute Respiratory, Severe Acute Respiratory Syndrome, Sperm, Syndrome, Transcription, Virus

Written by
Suchandrima Bhowmik
Suchandrima has a Bachelor of Science (B.Sc.) degree in Microbiology and a Master of Science (M.Sc.) degree in Microbiology from the University of Calcutta, India. The study of health and diseases was always very important to her. In addition to Microbiology, she also gained extensive knowledge in Biochemistry, Immunology, Medical Microbiology, Metabolism, and Biotechnology as part of her master's degree.
Source: Read Full Article