ciprofloxacin shortage 2009

Researchers in the United States and the UK have conducted a study showing how the B.1.1.7 (Alpha) lineage of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) adapted to suppress host innate immune responses in lung epithelial cells.
The SARS-CoV-2 virus is the agent responsible for the coronavirus disease 2019 (COVID-19) pandemic that continues to threaten global public health and has now claimed the lives of more than 3.74 million people worldwide.
Nevan Krogan from the Quantitative Biosciences Institute (QBI) Coronavirus Research Group in San Francisco, California, and colleagues found that B.1.1.7 isolates have dramatically increased levels of subgenomic RNA and increased expression of innate immune antagonists.
“Our data reveal how the SARS-CoV-2 B.1.1.7 lineage has adapted to the host by enhancing antagonism of the innate immune response, naproxen vs ultracet ” writes the team. “We propose that enhanced innate immune antagonism by the B.1.1.7 lineage contributes to its transmission advantage.”
The team says the findings have important implications for management of the ongoing pandemic and that expanding ongoing sequencing efforts to monitor subgenomic RNA levels will be critical in the identification of future SARS-CoV-2 variants of concern.
A pre-print version of the research paper is available on the bioRxiv* server, while the article undergoes peer review.
More about the B.1.1.7 variant
Following its initial detection in the UK in September 2020, the B.1.1.7 lineage of SARS-CoV-2 rapidly became the dominant variant worldwide.
Due to its increased transmissibility and potential threat to public health containment measures, the B.1.1.7 lineage was categorized as a variant of concern by Public Health England in December 2020.
The B.1.1.7 variant contains 23 mutations, eight of which lie in the viral spike protein. This spike mediates the initial stage of the infection process by binding to the human host cell receptor angiotensin-converting enzyme 2 (ACE2).
While research efforts have focused on this adaption of the spike protein for viral entry and immune escape, little attention has been paid to B.1.1.7 variant of concern (VOC)-defining mutations lying outside of the spike that may contribute to the transmission advantage.
Most B.1.1.7 mutations are found in the non-structural proteins Nsp3 and Nsp6, the accessory protein Orf8, and the nucleocapsid protein, all of which have previously been shown to modulate the innate immune response.
“Furthermore, it is not yet clear whether any of the B.1.1.7-specific mutations impact the expression levels of viral proteins,” says Krogan and colleagues.
“In sum, the impact of these additional mutations on viral replication, transmission and pathogenesis has not been characterized,” they write. “Innate immune responses can exert strong selective pressure during viral transmission and play an important role in determining clinical outcomes to SARS-CoV-2 infection.”
What did the current study involve?
Using the Calu-3 human lung epithelial cell model, the researchers investigated differences between B.1.1.7 isolates and “wave one” SARS-CoV-2 viruses.
Studies that employed unbiased abundance proteomics, phosphoproteomics, messenger RNA (mRNA) sequencing and viral replication assays showed that B.1.1.7 isolates are more effective at suppressing host innate immune responses in these airway epithelial cells.
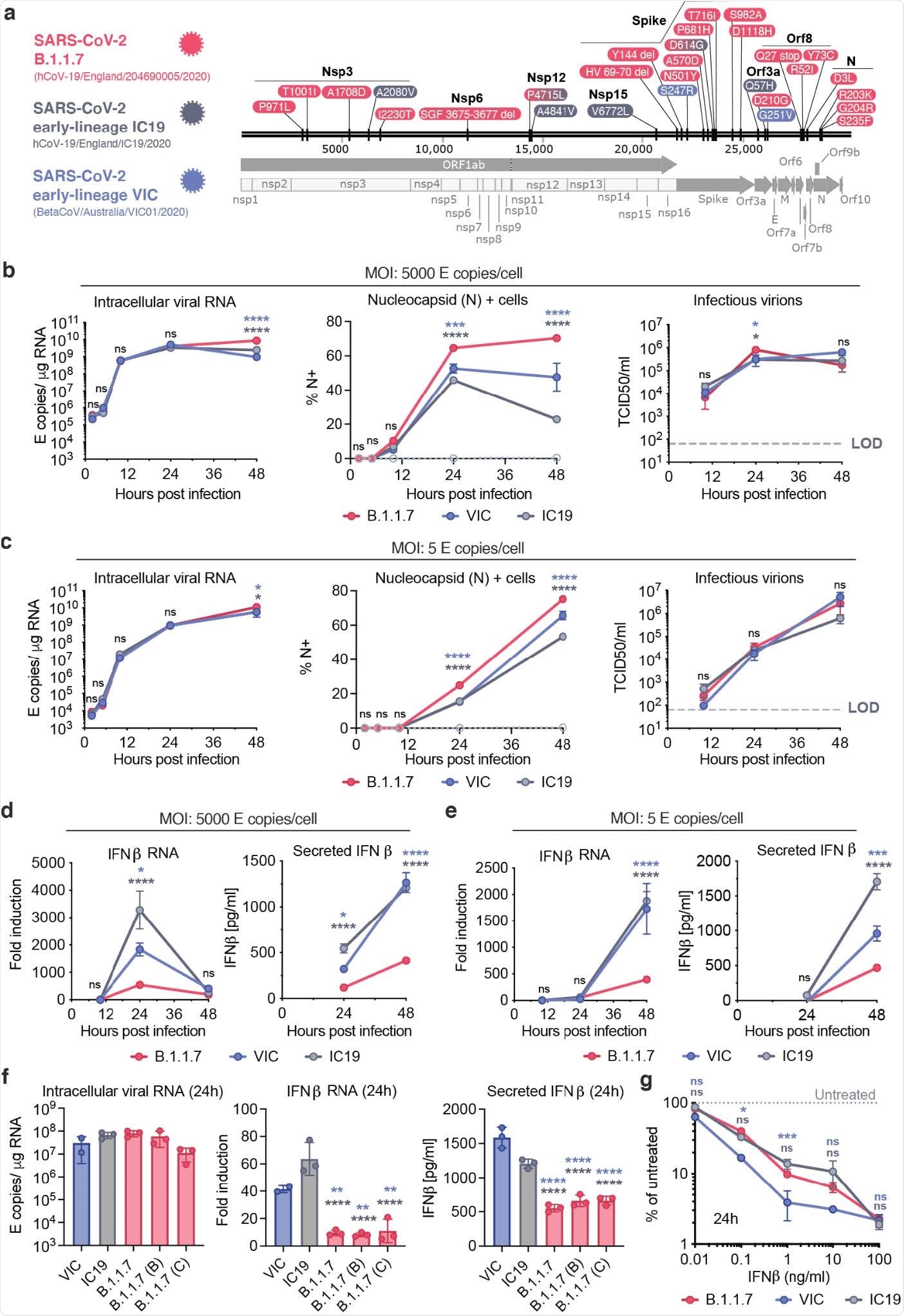
Strikingly, the team found that B.1.1.7 isolates have dramatically increased levels of subgenomic RNA and increased expression of the key viral innate antagonists Orf9b and Orf6.
“This remarkable and novel observation suggests evolution of B.1.1.7 nucleotide sequences that modulate specific single-guide RNA (sgRNA) production, and selection for increased sgRNA synthesis and protein expression,” writes Krogan and colleagues.
Inhibition of innate immunity by Orf9b may be regulated by the host innate response itself
The researchers found that expression of Orf9b alone suppressed the innate immune response through interaction with the mitochondrial protein TOM70, which is involved in the activation of mitochondrial antiviral signaling.
However, the study also revealed that Orf9b activation is regulated by phosphorylation.
“It is striking that Orf9b is not only enhanced in expression in B.1.1.7, but appears to be regulated by phosphorylation, which is, in turn, particularly repressed during B.1.1.7 infection,” say the researchers.
Krogan and colleagues say this suggests that inhibition of innate immunity by Orf9b is regulated by the host innate response itself.
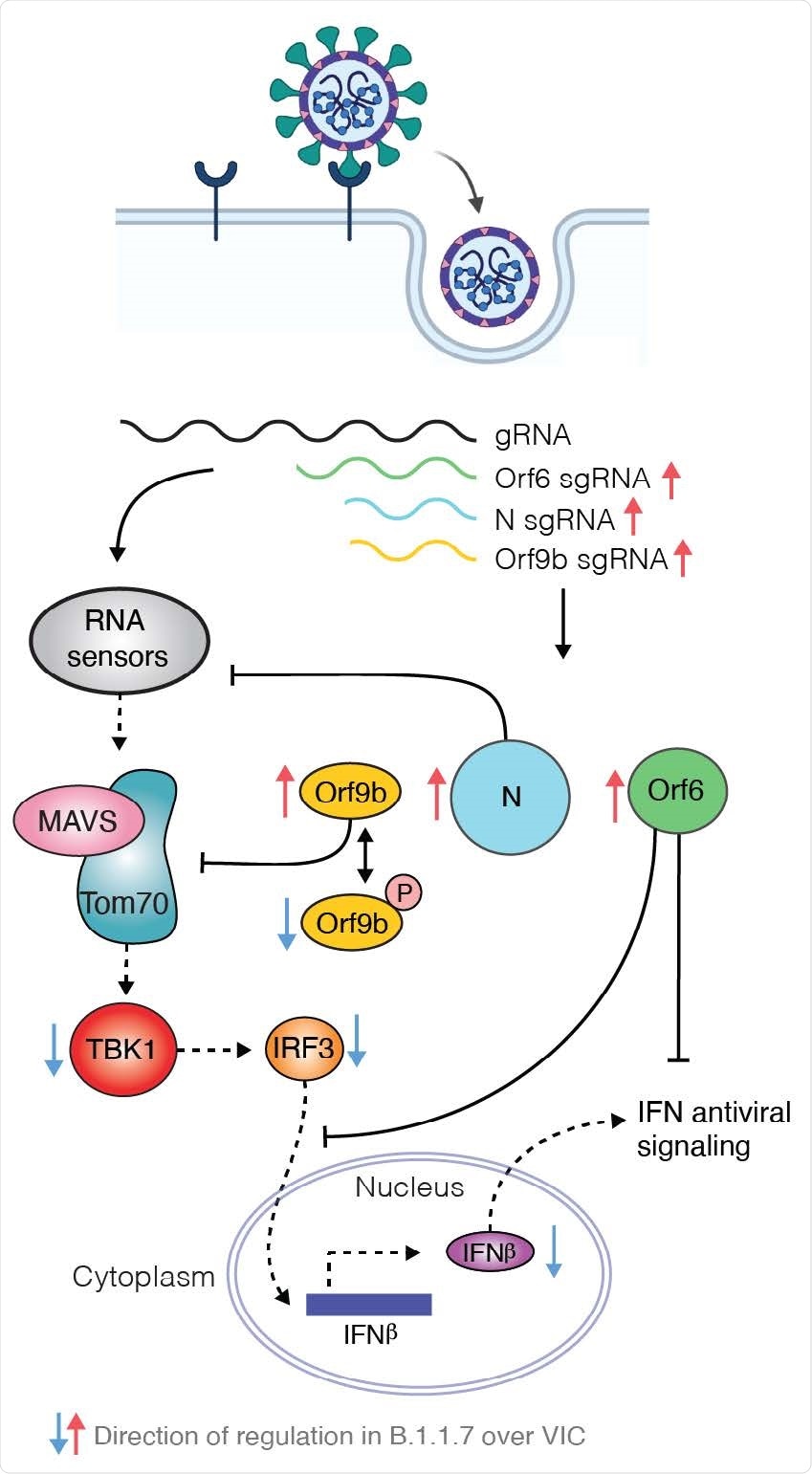
Unphosphorylated Orf9b was maximally active during the early stage of infection, which enabled effective innate antagonism and, therefore, viral production. However, as host activation began, Orf9b was phosphorylated and switched off to enable innate immune activation.
“Such an inflammatory switch may have evolved by coronaviruses to enhance transmission by increasing inflammation at the site of infection once virus production is high, leading to symptoms that promote transmission such as mucosal secretions and coughing,” suggests the team.
Monitoring subgenomic RNA levels will be critical in the identification of future variants
Krogan and colleagues say the findings have important implications for managing the ongoing COVID-19 pandemic.
“It is expected that expanding ongoing sequencing efforts to monitor subgenomic RNA levels will be critical in the identification of future SARS-CoV-2 variants of concern,” they write.
“Our findings highlight the importance of studying changes outside Spike to understand the phenotype of B.1.1.7, other current variants, and future variants, and to emphasize the importance of innate immune evasion in the ongoing process of adaptation of SARS-CoV-2 to a new host,” concludes the team.
*Important Notice
bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
- Krogan N, et al. Evolution of enhanced innate immune evasion by the SARS-CoV-2 B.1.1.7 UK variant. bioRxiv, 2021. doi: https://doi.org/10.1101/2021.06.06.446826, https://www.biorxiv.org/content/10.1101/2021.06.06.446826v1
Posted in: Medical Research News | Disease/Infection News
Tags: ACE2, Angiotensin, Angiotensin-Converting Enzyme 2, Cell, Coronavirus, Coronavirus Disease COVID-19, Coughing, Enzyme, Evolution, Gene, Gene Expression, Genome, Genomic, Immune Response, Inflammation, Intracellular, Nucleotide, Pandemic, Phenotype, Phosphorylation, Protein, Protein Expression, Proteomics, Public Health, Receptor, Research, Respiratory, RNA, SARS, SARS-CoV-2, Severe Acute Respiratory, Severe Acute Respiratory Syndrome, Spike Protein, Syndrome, Virus

Written by
Sally Robertson
Sally first developed an interest in medical communications when she took on the role of Journal Development Editor for BioMed Central (BMC), after having graduated with a degree in biomedical science from Greenwich University.
Source: Read Full Article