buy online herbal soma supreme suppliers without prescription

Histidine is an amino acid derived from proteinaceous dairy- and meat-based products. As one of 20 amino acids, it pays an important role in the body. This article examines histidine metabolism and the consequences this has on our health.
 Image Credit: Raimundo79 / Shutterstock
Image Credit: Raimundo79 / Shutterstock
Histidine structure
Histidine is an amino acid which can be obtained through hydrolysis of proteins. An abundant source of histidine is hemoglobin, where to buy generic xenical canadian pharmacy without prescription which contains 8.5%. The human body is unable to synthesize this amino acid, so it must be obtained through dietary means.
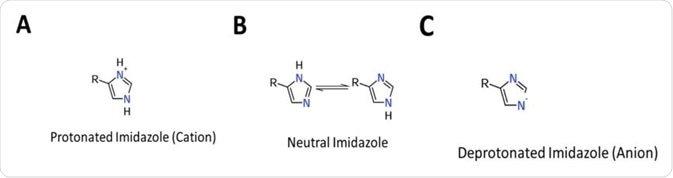
It readily ionizes within the body’s physiological pH range; this makes histidine a frequent participant in enzyme-catalyzed reactions as the protonated form can serve as a general acid, whilst the deprotonated form can serve as a general base (See Figure 1).
Furthermore, the basic nitrogen atom of histidine can function as an electron pair donor, and therefore readily participate in chemical reactions, through the formation of bonds with electron poor atoms.
Histidine function
Aside from is biochemical properties, histidine has many systemic functions in the body:
- Aids memory and cognitive function.
- Precursor to Histamine, the local mediator of allergic reactions. Histamine is vasoactive – it increases the diameter of blood vessels to improve blood flow.
- Removes surplus heavy metals and protects from radiation.
- Aids digestion by stimulating gastric juice production in the stomach.
- Increases the effectiveness of cancer drugs.
Histidine metabolism
All amino acids, including histidine, can be utilised for the purposes of metabolic energy production. In this process, amino acids are broken down to CO2 and H2O; this typically accounts for 10-15% of the metabolic energy generated by animals.
Alternatively, they can be used for gluconeogenesis. This is the metabolic pathway that generates glucose from non-carbohydrate compounds.
To achieve this, amino acids undergo degradation whereby loss of their functional amino group (NH2) yields different products called alpha-ketoacids; there are 7 of these in total.
Alpha-ketoacids are common metabolic intermediates and they can be categorised according to the point at which they enter the metabolic cycle:
- Gluconeogenic alpha-ketoacids (5 in total) are those which feed into the citric acid cycle and function as precursors of gluconeogenesis. The amino acids that yield these are subsequently termed gluconeogenic.
- Ketogenic alpha-ketoacids (2 in total) are those which feed into ketone bodies, fatty acids and isoprenoid production. The amino acids that yield these are subsequently termed ketogenic.
Histidine is a gluconeogenic amino acid. It is degraded by conversion to glutamate, and then oxidised to a-ketoglutarate by glutamate dehydrogenase.
Histidine is converted to glutamate in a 4-step process. It is first deaminated (the process by which the amino group is removed), and then hydrated.
Following this, pentameric ring structure of histidine, called imidazole, is cleaved to form a compound called N-formiminoglutamate.
The formimino group is then transferred to tetrahydrofolate (THF), forming the a-ketoacid glutamate and formiminotetrahydrofolate. This is catalysed by the enzyme glutamate formiminotransferase cyclodeaminase (FTCD). These steps are illustrated in Figure 2.
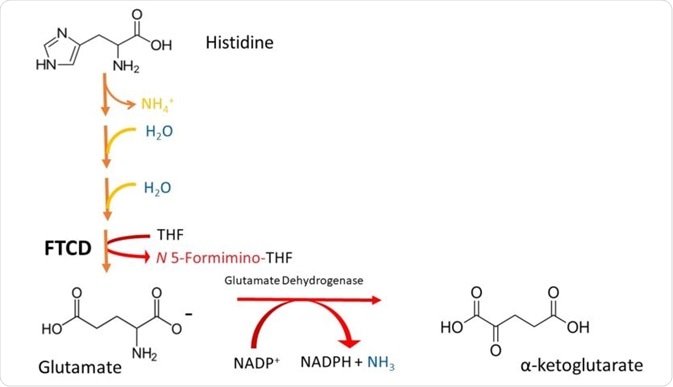 Figure 2. A simplified scheme depicting the degradation of Histidine to a-ketoglutarate. Note that histidine is deaminated (loss of NH4), then it is hydrated (addition of H2O in 2 successive steps), and its imidazole ring is cleaved to form formiminoglutamate. The formimino group is then transferred to THF, to produce glutamate and N5-formiminotetrahydrofolate by FTCD.
Figure 2. A simplified scheme depicting the degradation of Histidine to a-ketoglutarate. Note that histidine is deaminated (loss of NH4), then it is hydrated (addition of H2O in 2 successive steps), and its imidazole ring is cleaved to form formiminoglutamate. The formimino group is then transferred to THF, to produce glutamate and N5-formiminotetrahydrofolate by FTCD.
THF is important in cells as it functions as a carrier of one-carbon (C1) compounds. Many cellular reactions involve the addition of a C1 unit to a metabolic precursor.
Biotin and S-adenosylmethionine are also C1-carriers. THF, however, is more versatile as it can transfer the carbon in different oxidation states – a property which allows it to be used widely in biochemical processes.
Histidine biosynthesis includes an intermediate in nucleotide biosynthesis
Histidine is one of 9 essential Amino acids. Essential amino acids are those which cannot be synthesized by the organism, or de novo; their synthetic pathways are present only in microorganisms and plants. In these species, histidine is synthesized from the sugar ribose and the nucleotide adenosine triphosphate. The process by which this occurs is called biosynthesis.
Five of histidine’s 6 C atoms originate from 5-phosphoribosyl- alpha-pyrophosphate (PRPP), a phospho-sugar intermediate that is also involved in the biosynthesis of purine and pyrimidine nucleotides. The histidine’s 6th carbon atom originates from ATP; the remaining atoms in ATP are eliminated as another intermediate in purine biosynthesis.
Condensation of ATP and PRPP yields phosphoribosyl-ATP. The observation of this compound in histidine biosynthesis has far-reaching implications. It supports the notion that early life was originally RNA, rather than DNA based.
Histidine plays an important role in enzymes where it functions as a nucleophile or a general acid or base. RNA also possesses these similar properties, suggesting histidine also play as role in RNA enzymes.
Consequently, histidine biosynthetic pathways may be the remnants of the transition throughout evolution, to more efficient, DNA-encoded, protein-based life forms!
Histidine metabolism is rather complex; it is at the interface of many biosynthetic and metabolic reactions in the cell. It has profound effects on human physiology – from cognition to allergic reactions. As an essential amino acid, it is important that humans derive histidine from their diets.
Sources
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC239417/pdf/600044.pdf
- https://pubchem.ncbi.nlm.nih.gov/compound/6274#section=Other-Spectra
- https://www.britannica.com/science/histidine#%20Diet%20and%20Health.
- https://www.nature.com/articles/s41586-018-0316-7
Further Reading
- All Biochemistry Content
- An Introduction to Enzyme Kinetics
- Chirality in Biochemistry
- L and D Isomers
- Suzuki-Miyaura Cross-Coupling Reaction
Last Updated: Oct 17, 2018

Written by
Hidaya Aliouche
Hidaya is a science communications enthusiast who has recently graduated and is embarking on a career in the science and medical copywriting. She has a B.Sc. in Biochemistry from The University of Manchester. She is passionate about writing and is particularly interested in microbiology, immunology, and biochemistry.
Source: Read Full Article