buy benicar no prescription toronto

Researchers in Austria have conducted a study showing that individuals previously infected with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) exhibit a significantly more pronounced antibody response to one dose of Pfizer-BioNTech’s coronavirus disease 2019 (COVID-19) vaccine than previously uninfected individuals.
The team from the Medical University of Vienna says that an astonishing 20- to 100-fold increase in the median antibody level was shown for SARS-CoV-2 seropositive versus seronegative individuals.
The relative antibody increase among infection-naïve individuals was significantly higher following a second dose of the vaccine, but antibody levels still remained much lower than those of seropositive individuals.
“These results should stimulate discussion and research on whether individuals after previous SARS-CoV-2 infection would benefit from a two-part vaccination schedule or whether these currently much-needed second doses could be saved,” writes Helmuth Haslacher and colleagues in the European Journal of Clinical Investigation
.jpg)
Vaccine shortages raise the question of who should be prioritized
Vaccine supply shortages are posing a challenge to governments and policymakers in many countries around the world, buy online advair usa without prescription raising the question of who should be immunized against SARS-CoV-2 first.
Although there are no differentiated vaccination recommendations for previously infected and naïve individuals, preliminary evidence suggests that people who are seropositive for SARS-CoV-2 exhibit a more pronounced antibody response following a first vaccine dose.
“However, there are so far no data using Conformitè Europëenne- (CE) marked, automated platform assays for quantification of SARS-CoV-2 antibody responses,” says the team.
Most quantitative SARS-CoV-2 antibody assays detect antibodies generated against the viral spike protein – the structure that mediates the initial stage of the infection process when it binds to host cells.
In pre-vaccination times, these assays were developed because anti-spike antibodies were shown to correlate well with the presence of the neutralizing antibodies that develop following natural infection.
Assays are needed that distinguish between the previously infected and uninfected
The vaccines that are currently being rolled out, such as those developed by Pfizer-BioNTech, Moderna AstraZeneca, and Johnson & Johnson, all encode the viral spike protein to induce anti-spike antibodies.
Therefore, the presence of these antibodies could be indicative of either SARS-CoV-2 vaccination or previous infection.
“However, a further distinction can be made, since a natural infection with the SARS-CoV-2 virus induces antibodies against other viral compounds, for example, the viral nucleocapsid (NC), which can be detected by CE-marked immunoassays as well,” says Haslacher and colleagues.
“Therefore, even after vaccination with one of the mentioned vaccines, it is possible to distinguish those with a previous SARS-CoV-2 infection from individuals who were seronegative before their first shot.”
What did the researchers do?
The researchers compared antibody responses to Pfizer-BioNTech’s BNT162b2 vaccine in 12 previously infected individuals and 69 naïve individuals 21 days after they received their first dose.
Antibody responses to the viral spike protein (S) were assessed using the CE-labelled Roche SARS-CoV-2 S (Roche S) assay (which detects anti-NC antibodies) and the CE-labelled DiaSorin S1/S2 assay.
What did the study find?
After a single dose of BNT162b2, individuals with previous infection presented with markedly higher levels of anti-S antibodies than infection-naïve individuals.
The median level of anti-S antibodies as measured by the Roche S assay was 9078.5 Bioequivalent Allergy Units per mL (BAU/mL) among the previously infected individuals, compared with 79.6 BAU/mL among the naïve individuals. For the DiaSorin S1/S2 assay, the corresponding measurements were 1465.0 AU/mL and 63.7 0 AU/mL
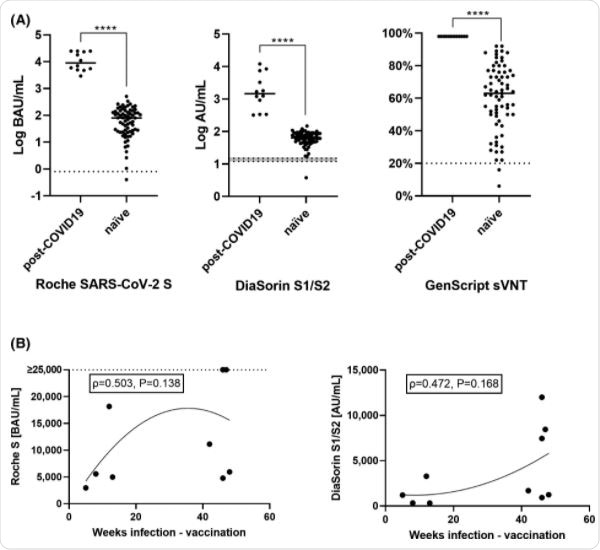
The team observed a trend for higher antibody responses in those with a more distant infection (occurring 42 weeks previously) for both the Roche S and the DiaSorin S1/S2 assay, although this relationship was not statistically significant.
Both the previously infected and infection-naïve groups showed increased antibody levels following a second vaccine dose, but levels were still significantly higher among the seropositive individuals.
Could currently much-needed second doses be saved?
“Our data clearly show that individuals with previous SARS-CoV-2 infection, as indicated by circulating antibodies against the viral nucleocapsid, respond considerably better after a single dose of BNT162b2 than naïve individuals did,” says Haslacher and colleagues.
“With these data, we hope to stimulate discussion and research on whether individuals after previous SARS-CoV-2 infection or known seropositivity would benefit from a two-part vaccination schedule or whether these currently much-needed second doses could be saved,” they write.
- Haslacher H, et al. Serum antibody response to BNT162b2 after natural SARS-CoV-2 infection. European Journal of Clinical Investigation, 2021. https://doi.org/10.1111/eci.13632
Posted in: Medical Research News | Disease/Infection News
Tags: Allergy, Antibodies, Antibody, Assay, Coronavirus, Coronavirus Disease COVID-19, Immunoassays, Protein, Research, Respiratory, SARS, SARS-CoV-2, Severe Acute Respiratory, Severe Acute Respiratory Syndrome, Spike Protein, Syndrome, Vaccine, Virus

Written by
Sally Robertson
Sally first developed an interest in medical communications when she took on the role of Journal Development Editor for BioMed Central (BMC), after having graduated with a degree in biomedical science from Greenwich University.
Source: Read Full Article