What underlies inconsistent clinical trials results for COVID-19 drugs?

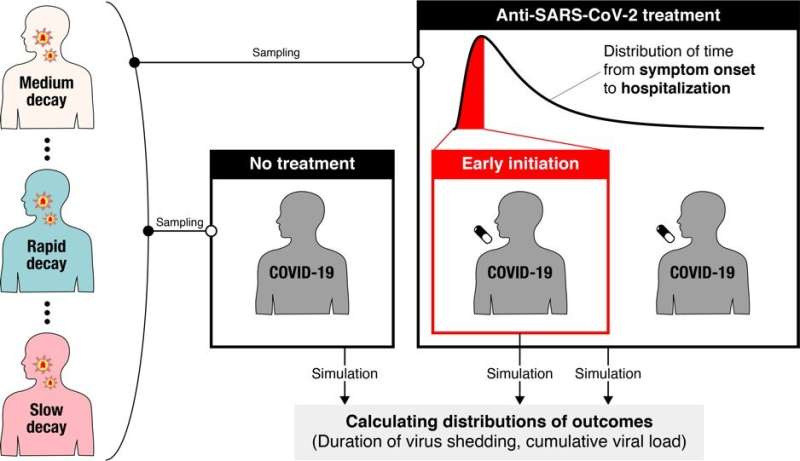
A new modeling study suggests that significant variation in virus dynamics from person to person may be a contributing factor to inconsistent findings reported in clinical trials for antiviral COVID-19 drugs. Recruiting trial participants shortly after symptoms begin could reduce the number of participants required to detect significant antiviral drug effects, according to Shoya Iwanami and Shingo Iwami of Nagoya University in Aichi, Japan, Keisuke Ejima of Indiana University, Indiana, USA, and colleagues, who present these findings in the open-access journal PLOS Medicine.
An effective antiviral drug for COVID-19 would have significant global health benefits. However, clinical trials that test candidate drugs have produced inconsistent results, perhaps due to flaws in the way the trials are designed.
To address this issue, Iwanami and colleagues first utilized a model of the dynamics of SARS-CoV-2—the virus that causes COVID-19—once it has infected a person. They combined the model with clinical data to examine how viral load (the amount of virus in a person’s throat) changes over time, and found significant variation in the rate of decline between patients. These differences may contribute to the inconsistent results reported in non-randomized clinical trials so far.
Exploring further, the researchers simulated potential findings of randomized clinical trials for COVID-19 drugs that successfully interrupt virus replication. They found that, even if a drug reduced viral replication by 95%, the associated randomized clinical trial would need to enroll more than 13,000 people to receive the drug being tested, plus the same number of people to receive a placebo for comparison, in order to detect statistically significant differences in viral load. In most cases, those numbers would be unreasonably large.
However, when the researchers altered the simulated randomized clinical trials so that participants were treated within one day of onset of their symptoms, they found that only up to about 600 participants were needed for each group. This suggests that randomized clinical trials for COVID-19 drugs could be improved by enrolling participants as soon as possible after symptoms appear, or by setting enrolment criteria based on the time that has passed since symptom onset.
The researchers note that future studies could employ more detailed models of SARS-CoV-2 dynamics in order to produce more reliable calculations of the numbers of participants needed for randomized clinical trials to produce consistent results.
Source: Read Full Article