Using AI to safely add people with red flags to clinical trials

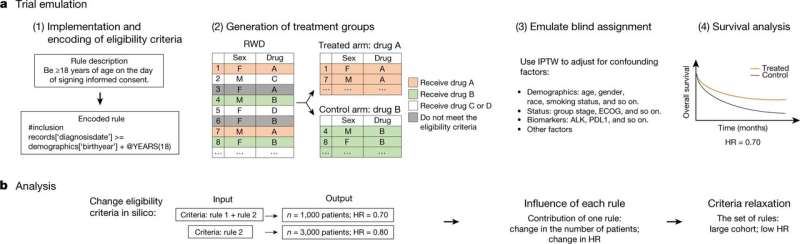
A team of researchers from Stanford University working with biotechnology corporation Genentech, has developed an artificial-intelligence based system that can safely add clinical trial participants that may have previously been excluded. They’ve published their findings in Nature; Chunhua Weng and James Rogers from Columbia University have published a News & Views piece on the work done by the team in the same journal issue.
In most countries, drugs must pass clinical trials before they are approved for patients to show that, in addition to providing the intended therapy, they are safe. But as the researchers with this new effort note, clinical trials in most places, including the U.S., suffer from one serious drawback—the people that are administered drugs in the clinical trials are specially selected. Most clinical trials, for example, do not allow pregnant women. And most have age requirements. Also, most do no allow those with conditions other than those that are being tested. This filtering process reduces the available pool of possible volunteers, and also unnecessarily excludes many people who may benefit from the therapy. The researchers with this new effort have sought to overcome this problem by building an AI-based system that can safely include more people in clinical trials.
Source: Read Full Article