The influenza virus and its influence on blood stem cells and coagulation

Virus-induced respiratory infections can become life-threatening. A team at the Paul-Ehrlich-Institut, together with researchers in Heidelberg, has discovered that flu virus infection limited to the lungs also leads to the activation of blood stem cells and the increased formation of platelets. Platelets can cause thrombosis, as has been shown in severe cases of COVID-19. The messenger substances interleukin-1 and interleukin-6 are involved in the activation. Cell Reports chronicles the results in its online edition from October 4, 2022.
Flu outbreaks of varying intensity occur every year in the winter months. They are caused by influenza viruses. Severe cases of influenza infection are associated with a derailment of the immune system, a cytokine storm with an excessive release of messenger substances (cytokines), and damage to lung cells. Infection can lead to blood vessel leaks and cause thromboses. These reactions are similar to severe cases of COVID-19 caused by the SARS-CoV-2 coronavirus. When do severe cases develop and what processes occur when this happens? Many details are unknown.
Complications of severe flu cases include both a reduced and an increased number of platelets in the blood, which can be associated with an increased chance of thrombosis. Researchers led by Professor Ute Modlich, head of the research group “Gene Modification in Stem Cells” at the Paul-Ehrlich-Institut, have worked in a research network with scientists at the German Cancer Research Centre (DKFZ) Heidelberg and the University of Heidelberg on the relationship between a flu virus infection (H1N1) and the formation of blood or platelets. H1N1 influenza viruses are among the influenza viruses that circulate annually in Germany.
Infection of the lungs with influenza viruses and its influence on blood formation
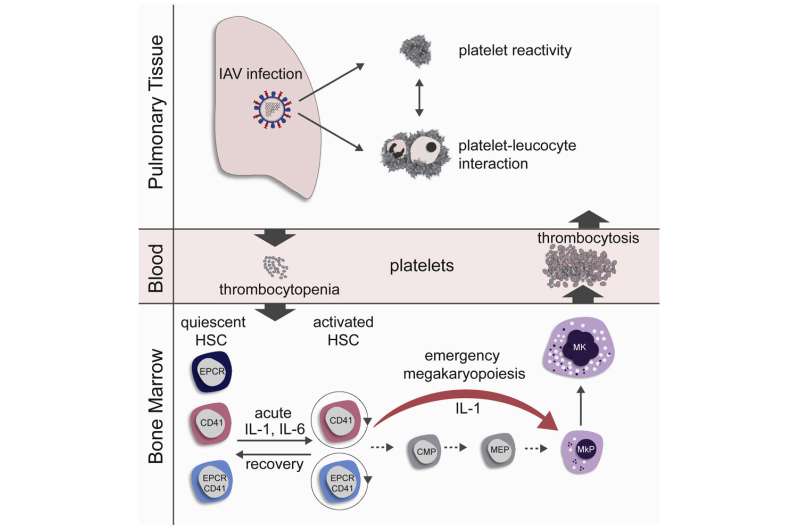
All blood cells and thus also all platelets are renewed by stem cells that can develop into blood cells (hematopoietic stem cells, HSCs). These cells exist in a resting state in the bone marrow. The fate of the HSCs is strictly regulated between rest, self-renewal and differentiation in order to ensure lifelong hematopoiesis.
In order to investigate the influence of influenza infection on blood formation, mice were infected intranasally with influenza viruses and their blood stem cells were subsequently investigated with regard to differentiation and cell cycle activation. In the first three days of acute influenza infection, the platelets initially decreased (thrombocytopenia), but then rose rapidly in the blood to levels above physiological levels (thrombocytosis). These rapidly produced platelets had an immature appearance (phenotype) and were more rapidly activatable (hyper-reactive).
An increased number of blood stem cells were found in the maturation process just two days after infection (G1 and S/G2/M cell cycle phase). The activation of the blood stem cells correlated positively with the viral lung titer, i.e., the more viruses had attacked the lungs, the more blood stem cells were activated. Infections with reduced influenza doses delayed stem cell activation, but could not prevent it. In the regeneration phase, the blood stem cells returned to the resting phase. This occurred faster in vaccinated mice than in other groups.
Blood stem cells with typical markers for platelet precursors
In order to clarify the question of how platelets can be produced so quickly, the research team took a closer look at the phenotype of the activated blood stem cells and found that a subset of the blood stem cells already carried typical markers of platelet precursor cells (megakaryocytes). Blood stem cells with this surface phenotype differentiate directly into megakaryocytes and produce platelets. They skip several preceding stages. The research group demonstrated through in vitro lineage tracing and bone marrow transplants that this group of blood stem cells rapidly multiply in the bone marrow after influenza infection. These newly produced platelets are larger and more immature in appearance than ordinary platelets and tend to activate more rapidly, which can lead to a higher risk of blood clots in the lungs.
The process of rapid differentiation of megakaryocytes has indeed already been described as emergency megakaryopoiesis, which occurs in response to systemic inflammations or infections. So far, however, no association with local viral respiratory diseases has been suspected. Although the influenza virus infection in mice was restricted to the respiratory tract, increased levels of the cytokines interleukin-1 (IL-1) and interleukin-6 (IL-6) were found in the bone marrow of infected mice. Using two groups of knockout mice, one with the IL-1 receptor switched off and one with the IL-6 receptor switched off, the research group demonstrated that these cytokines contribute decisively to the activation of the blood stem cells and to emergency megakaryopoiesis in influenza infections.
Source: Read Full Article