Team develops village in a dish system to transform stem cell research
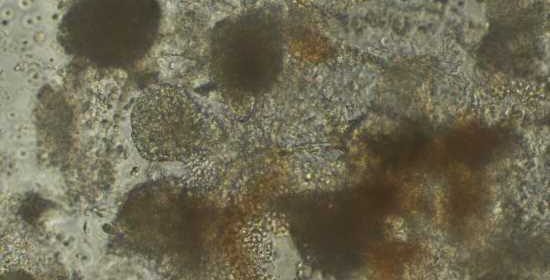
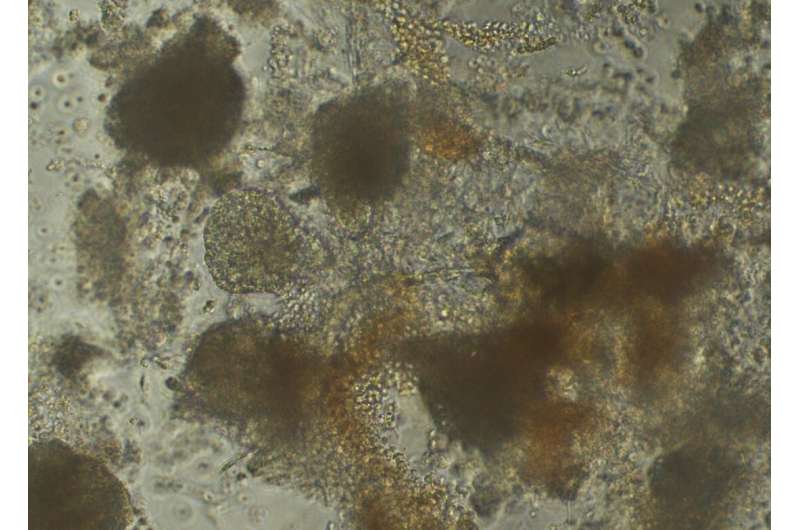
Studying stem cell samples from hundreds of individuals in the same culture dish, using a method developed at the Garvan Institute of Medical Research, could have transformative potential for personalized treatments and the study of complex human traits.
Stem cells can develop into many different cell types in the body and allow scientists to study diseases and test drugs in human cells. A major hurdle, though, is the expense and time required since these studies call for samples from hundreds—or even thousands—of donors.
The Garvan team’s innovative “village in a dish” system can accelerate this research by growing and studying stem cells from large numbers of donors simultaneously, in the same culture dish, making studies up to 100 times more efficient.
“Our village model provides a powerful way to understand how genetic differences between people influence health and disease,” says Professor Joseph Powell, Director of Cellular Science at the Garvan Institute, Director of the UNSW Cellular Genomics Futures Institute and senior author of the study. “Even though we share the majority of our DNA, variations in our genes lead to unique traits and responses. The village system captures this diversity at a large scale, revealing how genetic differences between people affect the complex mechanisms underlying biology and disease.”
Previous studies in population genomics have relied on a technique called bulk RNA sequencing to assess gene expression, which effectively averages the varied expression of different cell types into a single measure. This masks potential differences between individual cells or cell types and can provide an incomplete or biased view of gene expression in the sample, potentially leading to inaccurate or misleading conclusions.
To overcome these challenges the researchers developed the “village” approach, where stem cells from multiple donors are cultured in a single dish and analyzed using a technique called single-cell sequencing. Their findings show that the method retains the key features of the individual cultures. This provides an efficient solution for scaling experiments to the vast sample sizes required to give an accurate snapshot of the population.
A powerful setting for the study of human biology
The village-in-a-dish method enables large-scale studies of human organ systems that are normally inaccessible. Generating organ-specific cells from many individuals removes the need to obtain living tissue samples of those organs from donors and overcomes the limitations of studying only a few individuals’ cells. For example, it could reveal insights into cardiac conditions by generating heart cells from thousands of people, which would be impossible with direct tissue sampling.
“Medical research often relies on animal models, which are not perfect physiological proxies for humans. Induced pluripotent stem cells, which can be generated from a patient blood sample, provide the complete DNA profile of the patient and then can be differentiated into almost any cell type in the human body. From a basic science perspective, the stem-cell-based system is a powerful means to gain insights into the relationship between human genetics and biology that can’t be achieved with animal models,” says Professor Powell.
From culture dish to the clinic
This method will enable fast-tracked clinical trials to predict how groups of patients may respond to drugs, enabling the discovery of new treatments.
“The translational potential of this research is particularly compelling. By studying cells from many individuals at once, we can identify genetic factors that influence disease and treatment response at an unprecedented scale,” says Dr. Drew Neavin, first author of the paper and postdoctoral scientist at Garvan.
“An example is assessing cardiac toxicity, or Lewy body accumulation in Parkinson’s disease across hundreds of cell lines derived from patients. Rather than reflecting single individuals, such studies would reveal shared and distinct responses among genetic groups. The insights could then inform clinical trials by pre-selecting patient populations likely to benefit. Overall, this approach could transform our ability to translate stem cell science into precision treatments,” Neavin says.
The study was published in the journal Nature Communications.
It is part of a large collaborative body of work at the intersection of population genomics and stem cell research, with research partners from the University of Queensland, the University of Melbourne and the Menzies Institute.
“We look forward to seeing how our village system is used to gain unprecedented insights into the genetic basis of complex traits and diseases, translating to precise diagnostics, tailored treatments and better health for all,” says Professor Powell.
More information:
Drew R. Neavin et al, A village in a dish model system for population-scale hiPSC studies, Nature Communications (2023). DOI: 10.1038/s41467-023-38704-1. www.nature.com/articles/s41467-023-38704-1
Journal information:
Nature Communications
Source: Read Full Article