TAVR is an affordable and effective strategy for treating aortic stenosis patients

Minimally invasive, catheter-based transcatheter aortic valve replacement (TAVR) has revolutionized treatment of aortic stenosis (AS), and both American and European guidelines have approved its use. However, there is a perception that TAVR is more expensive than surgical aortic valve replacement (SAVR). A budget impact analysis comparing the two options now shows that TAVR is an affordable and effective strategy for the treatment of AS. The study appears in the Canadian Journal of Cardiology.
More than 3% of elderly adults suffer from advanced AS, with the majority of patients experiencing symptoms. Left untreated, AS has a poor prognosis and survival rate. Symptomatic patients develop chest pain, breathing difficulty, and fatigue, leading to diminished health-related quality of life. AS is traditionally treated with SAVR, but the use of TAVR is increasing. Patients who were previously considered inoperable have better survival with TAVR compared to medical therapy. In contrast, patients at high and intermediate surgical risk have equivalent outcomes when undergoing either TAVR or surgery.
“Although the cost-effectiveness of most transcatheter valve interventions is well studied, given the incremental costs associated with TAVR, the affordability from the hospital payer’s perspective was unknown,” explained coauthor Derrick Y. Tam, MD, Ph.D., Division of Cardiac Surgery, University of Toronto, Toronto, ON, Canada. “As low-risk AS patients likely represent the majority of severe AS patients requiring intervention, understanding the cost impact of treating more patients with TAVR becomes critically important for health policy and resource planning.”
“Traditionally, patients with severe AS were treated with SAVR through open heart surgery,” added coauthor Hamid Sadri, PharmD, MSc, MHSc, Medtronic Canada, Brampton, ON, Canada. “Because there is a perception that TAVR is expensive, hospital management often hesitates to increase funding for TAVR or reallocate some of the open heart surgery funding to TAVR cases. We hypothesized that despite the higher upfront procedural cost, the total cost of managing AS patients with TAVR was not substantially more than SAVR.”
Studies have shown that low-risk AS patients who are treated with TAVR have fewer procedure-related complications and adverse events and use less healthcare resources. To understand the incremental cost to hospitals in shifting low-risk AS patients from SAVR to TAVR at various risk levels, the researchers developed a budget impact model to estimate the one-year total cost of treating and managing low-risk AS patients from a hospital payers’ perspective.
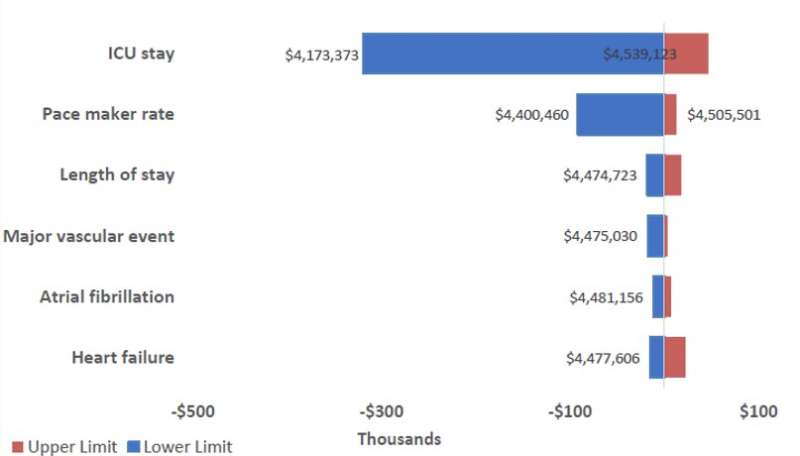
Then, researchers used a scenario analysis to estimate the total cost of care for various uptake rates of TAVR for a hypothetical center with 100 low-risk AS patients. This showed that increasing the use of TAVR from baseline (10% of patients) to 50% and 70% had a small impact on the hospital budget (increases of 3% and 4.5% respectively). Furthermore, in the first year of managing these patients, the upfront cost of the TAVR procedure was partially offset by the reduced cost of adverse events. The main contributors to the cost difference were TAVR ICU length of stay, new permanent pacemaker implantation rate, and total hospital length of stay. The average total cost of managing a low-risk AS patient for one year with TAVR (CAN$45,897) represents a nominal increase compared to SAVR (CAN$42,659).
“Our study demonstrates the importance of incorporating the total cost of care as opposed to a narrow focus on episodic or procedural costing,” noted Dr. Sadri. “The opportunity cost of not optimizing resource utilization often goes beyond a departmental silo and is a useful tool for hospital management.”
The researchers concluded that while survival rates do not vary, studies have shown that TAVR is associated with more rapid improvement in quality of life compared to SAVR, and that these findings persist to one year.
“As such, it is very reasonable that TAVR may be preferential to patients after a discussion of the risks and benefits. Similarly, the nominal incremental in hospital cost, can be offset by improved efficiencies in the system. Reduced overall length of hospital stay and ICU stay can relieve pressure on hospital bed capacity for optimizing resource use,” noted Dr. Tam.
In an accompanying editorial Fiona Clement, Ph.D., and Derek Chew, MD, MSc, both of the Cumming School of Medicine, University of Calgary, Calgary, AB, Canada, welcome the study as an important contribution to support evidence-informed policy decisions about the adoption and balance between TAVR and SAVR. They point out that the true value of the work is making the model underlying the analysis openly available to decision-makers and researchers globally.
“This model represents a significant infrastructure. By making the model open access, the required investment of time and human resources need not be duplicated as decision-makers face similar questions about shifting from SAVR to TAVR. Further, the model can be responsive as science continues to develop.”
However, Dr. Clement and Dr. Chew warn that costs alone should not drive the discussion of technology adoption and implementation. Recognition of other important factors should be taken into consideration, such as whether an intervention is immediately lifesaving; impact on quality of life; number of people eligible; vulnerable patient populations (e.g., children or the elderly); underlying baseline health; likelihood of the treatment being successful; and its impact on equality of access to therapy.
“Looking through this lens with respect to TAVR funding underscores the complexity of the decision-making process in the context of finite resources and the associated opportunity costs. These decisions will only become more challenging as innovation continues, often coming at increased cost,” Dr. Clement and Dr. Chew concluded.
Source: Read Full Article