Surgical sealing made better with robust thermosensitive bioadhesives
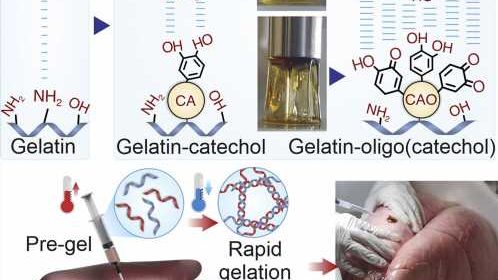
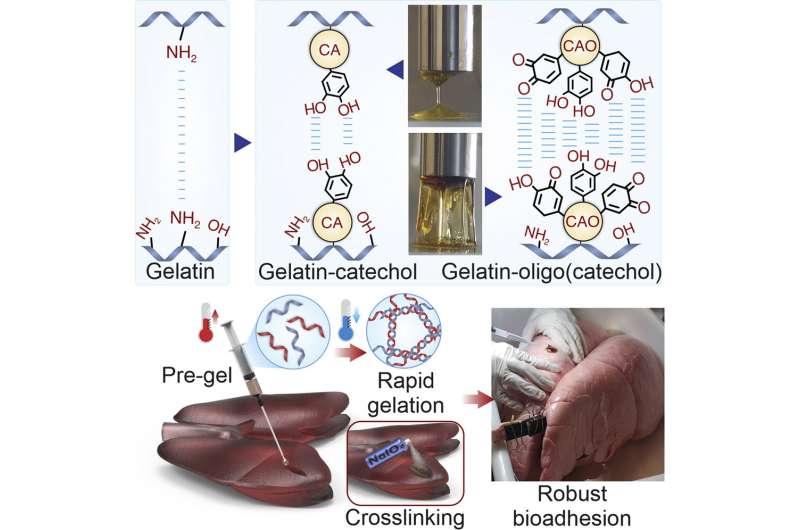
As part of a collaborative effort, scientists from the Terasaki Institute for Biomedical Innovation (TIBI) have employed inventive chemistry to produce an injectable biomaterial with significantly improved adhesive strength, stretchability, and toughness.
This chemically modified, gelatin-based hydrogel had attractive features, including rapid gelation at room temperature and tunable levels of adhesion. This custom-engineered biomaterial is ideal as a surgical wound sealant, with its controllable adhesion and injectability and its superior adherence to a variety of tissue and organ surfaces.
In order to provide closure for surgical wounds, the material must provide effective sealing on wet, slippery tissue surfaces, which vary in shape and could possibly involve tissue movement (an expanding lung, for example) or crumbly textures. The application and effectiveness of the sealant must also be within a suitable timeframe for surgical procedures.
Standard methods of suturing and stapling can be ineffective and time-consuming and can result in increased blood loss. Other methods using fibrin-based bioadhesive sealants are costly, exhibit insufficient adhesion, and can be prone to viral transmission.
Commercial gelatin-based wound dressings, although offering biocompatibility, low cost, and hemostatic effectiveness, lack adhesive strength due to inherent brittleness. Previous efforts to address poor adhesion problems have been made via functionalization with catechol, a naturally occurring compound that can impart adhesive capabilities when bound to gelatin. However, the small number of binding sites on the gelatin results in limited level of adhesion that can be enabled by catechol functionalization.
The researchers chose caffeic acid (CA), a catechol-containing compound found in coffee and olive oil, to increase the tissue adhesion efficacy of gelatin. They first oxidized CA to yield CA oligomers (CAO) which involve a small number of repeating catechol units. Coupling these CA derivatives to gelatin amplified chemical binding of catechol groups and boosted their adhesive function.
The engineered bioadhesive sealant had superior adhesive strength, stretchiness, toughness, and injectability, along with the ability for rapid gelation when applied to the wound site and showed stable adhesion in physiological conditions.
Furthermore, the sealants were designed to adhere to tissue in a selective manner. This is pivotal for an effective sealant, as there needs to be tight bonding at the sealant-tissue interface and non-binding on the opposite face of the sealant, which is exposed to the bodily environment.
Validation tests using the new sealant on wet collagen sheets, as well as burst pressure experiments to test the limits of its adhesive strength, demonstrated its effectiveness and proved contrary to previous reports of the detrimental effects of oxidative chemistry.
Experiments conducted on pig lung, heart, and bladder wounds showed that the new sealant had adhesive strength an order of magnitude higher than commercial gelatin-based sealants; the sealant also remained affixed to the tissue surface even after experimental scraping and twisting.
The new sealant was also shown to be biocompatible. In addition, it was shown to have drug loading and drug release capabilities and could promote antioxidant effects beneficial to wound healing. This versatile strategy can be adapted as a powerful approach to impart strong adhesion to other biomaterials.
“Our team has utilized manipulative and strategic chemistry to significantly improve adhesive strength and versatility in biomaterials,” said Ali Khademhosseini, TIBI’s Director and CEO. “This creates exciting possibilities for more effective surgical wound management in the clinic.”
The study is published in the journal Cell Reports Physical Science.
More information:
Hossein Montazerian et al, Injectable gelatin-oligo-catechol conjugates for tough thermosensitive bioadhesion, Cell Reports Physical Science (2023). DOI: 10.1016/j.xcrp.2023.101259
Journal information:
Cell Reports Physical Science
Source: Read Full Article