Study sheds light on how breast cancer cells evade immune surveillance and survive in lymph nodes
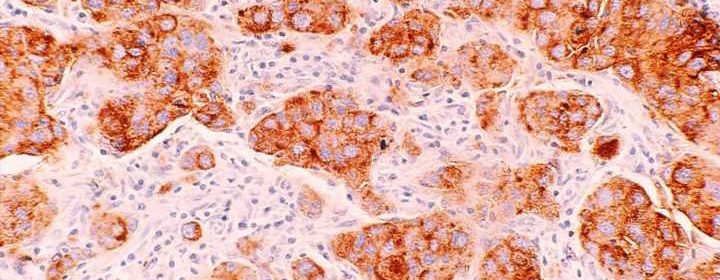
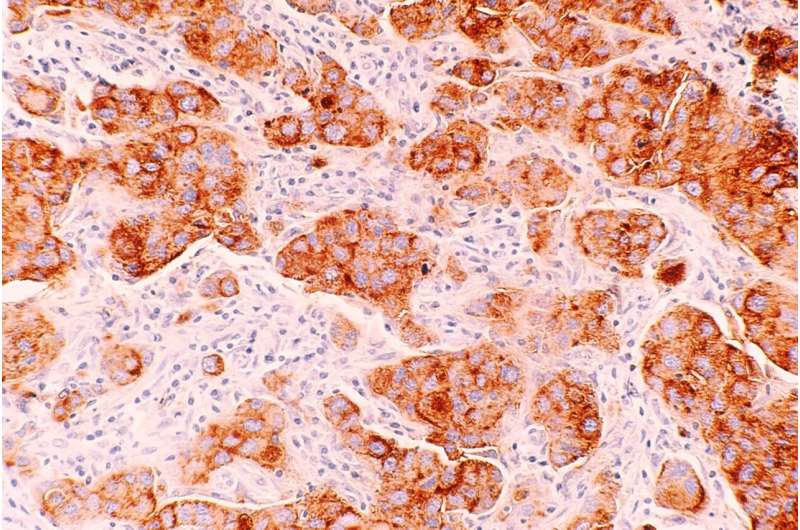
In breast cancer, nearby lymph nodes, which are part of the immune system, are usually the first site of cancer spread, and from here cancer cells can travel to other parts of the body, such as the brain, lung, liver, and bones.
New research led by investigators at Massachusetts General Hospital (MGH) reveals how cancer cells suppress anti-cancer immune responses in the lymph nodes to survive and spread, or metastasize.
The findings, which are published in the Journal of Experimental Medicine, could lead to new strategies on how to prevent this suppression and unleash the immune system to fight cancer.
In a breast cancer mouse model, the scientists analyzed and compared the gene expression patterns of individual cancer cells that were residing within breast tissue or had metastasized to lymph nodes. They also studied how immune cells acted in the presence of these cancer cells. Finally, the team examined published data on gene expression patterns in human breast cancer cells during lymph node metastasis.
These efforts revealed that some breast cancer cells in the lymph nodes of mice and humans exhibit elevated expression of genes that code for MHC class II (MHC-II) proteins, which—when present on the cell surface of antigen-presenting cells—are involved in initiating immune responses.
However, the MHC-II+ cancer cells lacked what are called co-stimulatory molecules typically present on antigen-presenting cells that alert immune cells of danger. As a result, the lymph nodes had higher numbers of tolerant immune cells and fewer activated immune cells.
Knocking out the gene for MHC-II in cancer cells reduced lymph node metastasis and the expansion of tolerant immune cells in mice, leading to prolonged animal survival.
On the other hand, overexpression of a protein that increases MHC-II expression worsened lymph node metastasis and caused excessive expansion of tolerant immune cells.
“Our findings have significant implications for developing effective treatments to target lymph node metastases, prevent cancer spread to other organs, and restore anti-tumor immunity in the tumor-draining lymph nodes,” says co–lead author Pin-Ji Lei, Ph.D., a postdoctoral research fellow in Radiation Oncology at MGH.
Co–senior author Timothy P. Padera, Ph.D., the 2021–2026 Rullo Family MGH Research Scholar and an associate professor of Radiation Oncology at Harvard Medical School, notes that additional research is needed to fully determine the clinical significance of the group’s findings.
“We need to see if cancer cell reprogramming by the lymph node microenvironment affects patients’ anti-cancer immune response,” he explains.
Additional co-authors include Ethel R. Pereira, Patrik Andersson, Zohreh Amoozgar, Jan Willem Van Wijnbergen, Meghan J. O’Melia, Hengbo Zhou, Sampurna Chatterjee, William W. Ho, Jessica M. Posada, Ashwin S. Kumar, Satoru Morita, Lutz Menzel, Charlie Chung, Ilgin Ergin, Dennis Jones, Peigen Huang, and Semir Beyaz.
More information:
Pin-Ji Lei et al, Cancer cell plasticity and MHC-II–mediated immune tolerance promote breast cancer metastasis to lymph nodes, Journal of Experimental Medicine (2023). DOI: 10.1084/jem.20221847
Journal information:
Journal of Experimental Medicine
Source: Read Full Article