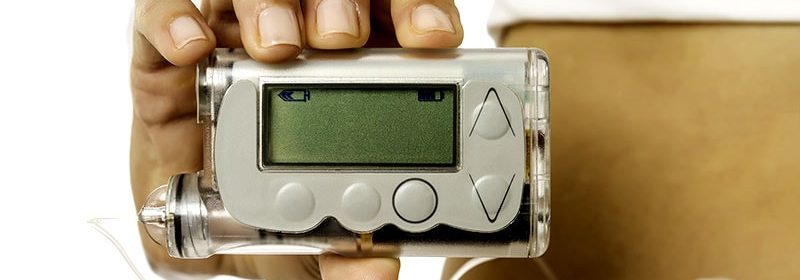
Skin Problems Common at Insulin Pump Infusion Sites
The study covered in this summary was published in The Lancet as a preprint and has not yet been peer reviewed.
Key Takeaways
Allergic sensitization at insulin pump sites is common.
Pump vs control sites had greater vessel density, fibrosis, fibrinogen, and inflammation including eosinophils, insulin-like-growth factor-1 (IGF-1), and transforming growth factor (TGF) β-3, as well as fat necrosis.
The tissue changes resulting from these skin responses could potentially lead to the infusion site failures commonly seen in clinical practice.
Why This Matters
Approximately 55% of diabetic ketoacidosis episodes in patients using insulin pumps are reportedly due to “pump/tubing” malfunctions.
Anecdotally, pump discontinuation is common after 20 years, owing to site failures, but just how frequently this occurs is unknown.
Little is understood about the cutaneous changes from chronic insulin infusion and how this may impact infusion site failure.
Study Design
Optical coherence tomography (OCT) was performed immediately before skin punch biopsy samples were collected at three sites: a “current site,” a “recovery site” ― used 3 days prior to biopsy ― and a “control” site never used for any insulin infusion or injection.
Analysis included 25 participants with a total of 75 skin sites.
Pump brands used were Medtronic, Tandem, and Omnipod, with steel, teflon, and Omnipod infusion sets used.
Key Results
Cutaneous symptoms at pump sites were common, with 93.3% of participants reporting itching and 76.7% reporting skin redness.
The OCT angiography microvascular maps showed increased blood vessels in both current and recovery pump sites vs control sites.
Statistical analysis showed a significant difference in vessel area density between pump and control sites (P < .0001) and between recovery and current pump sites (P < .001).
In histologic analysis, both current and recovery sites showed increased fibrosis, fibrinogen, inflammation, vascularity, and fat necrosis compared to control sites (all P < .001), with no significant differences seen between the current and recovery sites.
No eosinophils were observed in skin biopsy samples at the control sites, whereas eosinophils were identified in 73% of skin biopsy samples from the current sites (P < .01) and 75% of skin samples from the recovery sites (P < .01).
All study participants had eosinophils (range, 0–31/high power field; median, 4) identified in at least one current and/or recovery insulin infusion sites, located deep in the dermis near the interface with the fat.
There was no significant association between the type of insulin or pump brand and number of eosinophils.
Higher eosinophil counts were seen in patients using pumps for <10 years compared to those using pumps >20 years (P = .02).
Immunohistochemical staining also showed differences between current/recovery sites and the control site for insulin-like-growth factor-1 (IGF-1) and transforming growth factor β-3, but no difference with IGF1 receptor.
There were no differences in either OCT or histologic findings based on duration of pump use or with prior use of animal insulin.
Limitations
The sample size was relatively small.
The cohort consisted of adults who were mostly White, with normal weight and with excellent glycemic control.
The findings may not be generalizable to other populations, particularly younger patients.
Disclosures
The study was funded by the Leona M. and Harry B. Helmsley Charitable Trust.
Lead author Kalus has no disclosures, but one author reports grants and/or consulting fees from various sources, including Insulet, Medtronic, Dexcom, Abbott Diabetes, Roche, Bigfoot, and Lifescan.
This is a summary of a preprint research study, “Evaluation of Insulin Pump Infusion Sites in Type 1 Diabetes,” by Andrea Kalus, MD, of the University of Washington School of Medicine, Seattle, and colleagues. This study has not yet been peer reviewed.
For more news, follow Medscape on Facebook, Twitter, Instagram, and YouTube.
Source: Read Full Article