Seeking leukemia’s Achilles heel

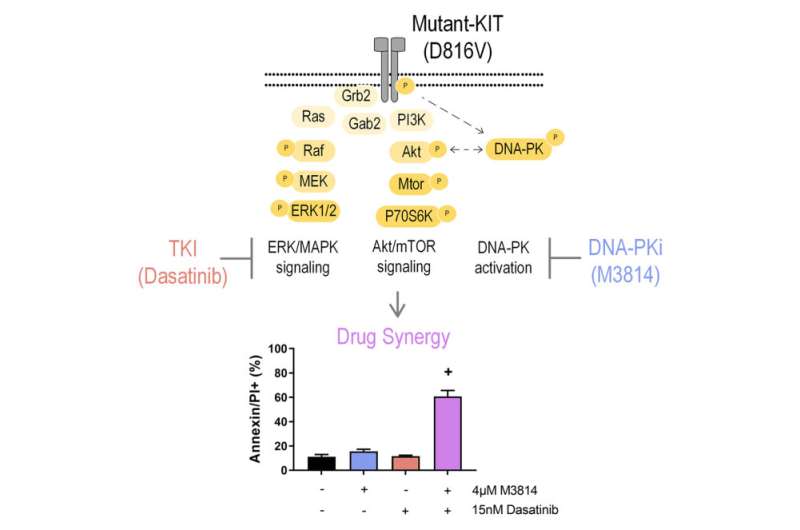
A team of researchers has discovered a potential therapeutic that can synergize with existing drugs to more effectively kill certain leukemia cells. The authors published their results in Molecular & Cellular Proteomics.
Acute myeloid leukemia is a cancer of developing immune cells. It can manifest in all individuals, including the elderly and children. Only 30% of patients survive beyond five years of diagnosis
Unlike cancers of solid organs, AML is found in bodily fluids, such as blood. Like passengers traveling to places all over the world on a high-speed train, AML cancer cells have access to every corner of the body via the blood.
To make things more difficult, “no two leukemias are alike,” Heather Murray, a postdoctoral researcher at the University of Newcastle and lead author of the study, said.
A patient’s prognosis can vary widely depending on genetic mutations found in AML cells. “Gene mutations present in a patient’s cancer have been used to predict aggressiveness of the disease and inform which treatment the patient should receive,” Murray explained.
Since the breakthroughs of the Human Genome Project, many cancer researchers have been focused on finding the cause of cancer inside patients’ DNA. However, because a cure for cancer remains elusive, Murray and her colleagues are diving deeper into tumor cell abnormalities that go beyond DNA.
“Our team has been working to develop precision therapies for AML,” Murray said. “This involves first identifying the specific features of different AML cells that drive them to grow and spread. We then use this information to try and tailor therapies that target these specific features.”
Murray continued, “A growing collection of studies are showing that analyzing the makeup of the cancer cells and how they communicate, such as proteomic and phosphoproteomic analyses, combined with the study of gene mutations, reveals much more.”
Phosphoproteomics is a technique that allows researchers to look at the modifications of proteins inside a cell. Specifically, this technique examines the phosphorylation status of a protein. Phosphorylation is known as a “cellular on-off switch.” Combined with other methods, phosphoproteomics takes cancer research to the next level, Nicole Verrills, an associate professor at University of Newcastle who oversaw the study, said.
The researchers used mouse cells that were engineered to have a mutation in a gene called KIT, which is found in approximately 1 in 20 AML patients.
Murray and her colleagues found that a protein called DNA-dependent protein kinase, known as DNA-PK for short, is always turned on in leukemia cells with the KIT mutation. So, they decided to try inhibiting both mutant KIT and DNA-PK to stop leukemia cell growth.
The researchers found that using a combination of the standard-of-care drugs and a DNA-PK inhibitor decimated the leukemia cells while having minimal impact on healthy cells. Murray said the team is optimistic that, by limiting side effects early on in the drug-discovery process, their approach will translate to a tolerable therapeutic.
The team previously found that the DNA-PK inhibitor also slows cancer growth in leukemia cells with a mutation in the gene FLT3. They hope a DNA-PK inhibitor could have broad therapeutic effects in many different kinds of leukemia and other cancers as well.
“These results mean that we can potentially treat more patients with similar types of therapy,” Verrills said. “It also expands the potential number of patients we could enroll in a clinical trial for this particular type of therapy.”
Clinical trials testing compounds similar to those used in this study in cancer patients have begun and are showing promising results.
Verrills and Murray have a vision of future precision therapy efforts that incorporates rapid genomic and phosphoproteomic studies as well as a large drug screen on an AML patient’s cancer cells before they receive any treatment. Verrills said this kind of testing would allow clinicians to predict the therapeutics to which an individual patient will or will not respond.
“This exciting study provides insight into different subtypes of AML,” Murray said “We have identified a potential new treatment approach for this devastating disease, which we hope to soon test in clinical trials.”
More information:
Heather C. Murray et al, Synergistic Targeting of DNA-PK and KIT Signaling Pathways in KIT Mutant Acute Myeloid Leukemia, Molecular & Cellular Proteomics (2023). DOI: 10.1016/j.mcpro.2023.100503
Journal information:
Molecular & Cellular Proteomics
Source: Read Full Article