Scientists identify new pathway activated by interferon-gamma that leads to tumor cell death
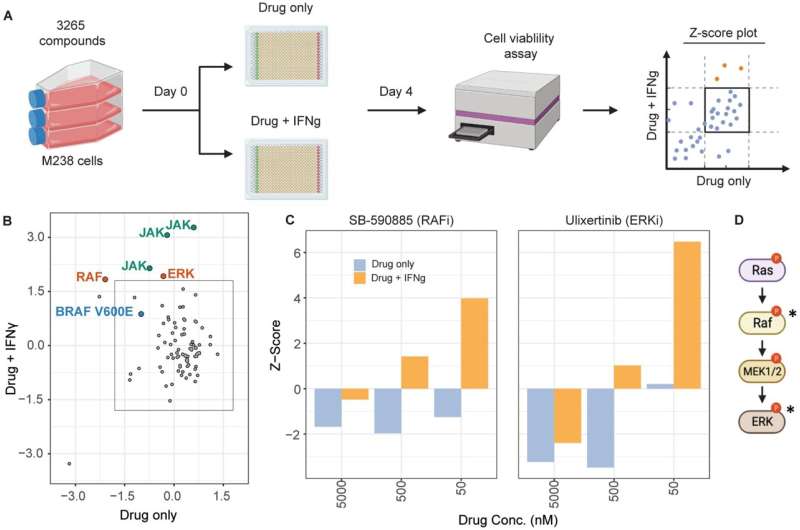
Researchers at the UCLA Jonsson Comprehensive Cancer Center have identified a new role for a protein called extracellular signal-regulated kinase (ERK) in a pathway activated by interferon-gamma that can trigger cells to self-destruct.
Researchers found that interferon-gamma signaling caused hyperactivation of ERK in human melanoma cell lines. The ERK protein, when hyperactive, causes stress in the cell, and this stress ultimately leads to cell death through specific proteins called DR5 and NOXA. Cell death could be prevented in 74% of these lines when ERK signaling was blocked. The study was published in the journal Molecular Cancer.
“ERK signaling is always active at a low level in melanoma cells and is important for tumor cell survival,” said Ameya Champhekar, an adjunct assistant professor of medicine at the David Geffen School of Medicine at UCLA, and first author of the study. “However, our data show that interferon-gamma causes overactivation of the ERK pathway, which triggers cell death.
“This establishes a new paradigm in the field that the overactivation of a pathway involved in oncogenic signaling is detrimental to cancer cells. This discovery sheds light on how interferon-gamma stops tumor cell growth and why it might not always work, helping us better understand how to overcome resistance.”
Interferon-gamma—an immune response–stimulating signaling molecule—helps activate the immune system to recognize and attack cancer cells. While it is known that interferon-gamma inhibits the growth of tumor cells, the exact ways it does this are not yet fully understood. Understanding how interferon-gamma works holds the potential for therapeutic targeting of this pathway and could rationalize the development of novel combination treatments.
The team used various screening techniques to understand how interferon-gamma affects melanoma cells and how it might help stop their growth. They performed chemical genomics and whole genome targeting CRISPR/Cas9 screens using patient-derived melanoma lines to uncover essential nodes in the interferon-gamma-mediated growth inhibition pathway.
They also used transcriptomic profiling to determine which cell death pathways were activated. Live imaging experiments coupled with apoptosis assays were used to confirm the involvement of these pathways in causing cancer cells to die when exposed to interferon-gamma.
Even though immunotherapy has revolutionized cancer treatment for those with advanced forms of the disease, it still only works in a small subset of patients. Interferon-gamma is an important weapon in the arsenal of T cells that attack tumor cells. Recent research shows that it can diffuse deep inside the tumor and has the potential to cause long-distance growth inhibitory effects on cancer cells. However, until now, it was unclear how its ability to stop tumor growth could be exploited for clinical benefit.
This new understanding of the interferon-gamma growth inhibition pathway is an important step in determining how to better target cancers that don’t respond well to immunotherapy.
More information:
Ameya Champhekar et al, ERK mediates interferon gamma-induced melanoma cell death, Molecular Cancer (2023). DOI: 10.1186/s12943-023-01868-x
Journal information:
Molecular Cancer
Source: Read Full Article