Researchers identify molecule that activates cell cycle to enhance cardiac tissue graft production
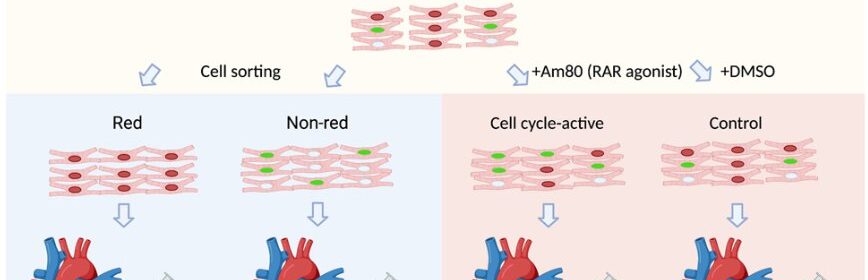
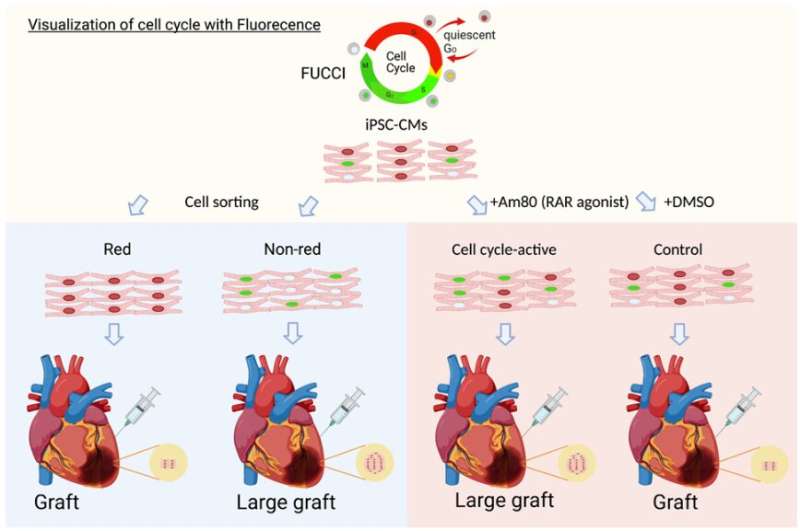
Assistant Professor Shunsuke Funakoshi, Associate Professor Yoshinori Yoshida, and their research team have identified Am80 as a molecule that activates the cell cycle in cardiomyocytes to enhance cardiac tissue graft production and engraftment.
For cardiac cell therapy, human pluripotent stem cell-derived cardiomyocytes (hPSC-cardiomyocytes) hold great promise for various applications, such as modeling heart diseases in a dish and generating cardiac tissues for transplantation. However, these cardiomyocytes often do not survive or integrate well following transplantation into a damaged heart, thus limiting their therapeutic effects.
To address this challenge, researchers focused on enhancing the engraftment potential of hPSC-cardiomyocytes. Previous studies have shown that the growth of the transplanted cells in the heart is closely linked to the cell cycle. For instance, increased cell cycle activation in cardiomyocytes has been associated with larger graft sizes and better improvement in cardiac functions. Researchers hope to enhance graft performance for cardiac cell therapy by exploring new methods to promote cell cycle activation in hPSC-cardiomyocytes.
The study, published in Stem Cell Reports, introduced the Fluorescence Ubiquitination-based Cell Cycle Indicator (FUCCI) system, a well-established technology that uses two fluorescent fusion proteins for monitoring cell cycle activation. The researchers applied the FUCCI system to human induced pluripotent stem cell-derived cardiomyocytes (hiPSC-cardiomyocytes) and measured their cell cycle activities during cardiac differentiation.
By separating these cells based on their FUCCI signals, the researchers identified differences in gene expression patterns at different cell cycle stages in vitro. Furthermore, they demonstrated the cell cycle activity of hiPSC-cardiomyocytes before transplantation to influence their in vivo proliferative potential following transplantation.
Using the FUCCI system for high-throughput screening, the researchers identified Am80, a retinoic acid receptor agonist, as a compound that effectively activates the cell cycle in hiPSC-cardiomyocytes. Treatment of hiPSC-cardiomyocytes with Am80 before transplantation significantly increased the size of grafts grown into injured mouse hearts. The study also revealed WNT signaling pathway activation—mediated by retinoic acid receptors RARA and RARB—as the underlying molecular mechanism of Am80-induced cell cycle activation.
Overall, this study demonstrates a link between cell cycle activity and graft size and functionality of hiPSC-cardiomyocytes. With Am80, the research team also provides an efficient method to activate the cell cycle in hiPSC-cardiomyocytes to increase graft size and improve functional outcomes for cell transplants to repair damaged hearts. These findings contribute to the development of regenerative therapies using hiPSC-cardiomyocytes and offer novel insights into how we could maximize their engraftment potential for clinical applications.
More information:
Manabu Kasamoto et al, Am80, a retinoic acid receptor agonist, activates the cardiomyocyte cell cycle and enhances engraftment in the heart, Stem Cell Reports (2023). DOI: 10.1016/j.stemcr.2023.06.006
Journal information:
Stem Cell Reports
Source: Read Full Article