Researchers develop new CRISPR-based tool for cancer diagnosis
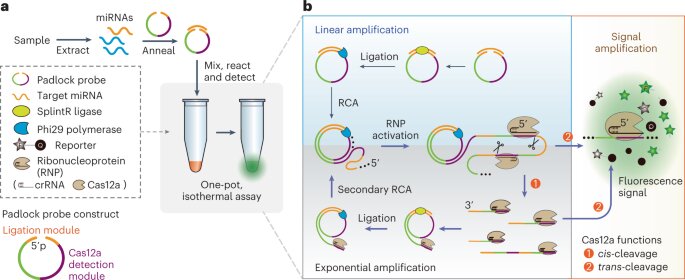
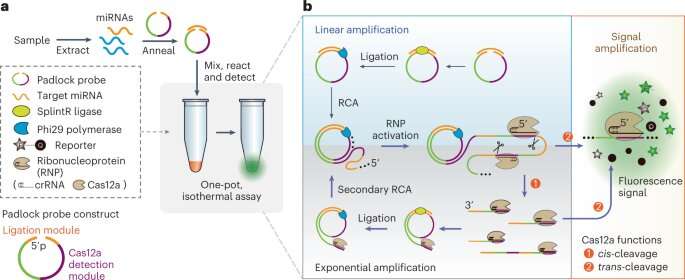
A team of University of Florida researchers has developed a promising new CRISPR-powered method for non-invasive blood tests that could help clinicians diagnose cancer at earlier stages.
The team demonstrated that their strategy is as effective as the widely used reverse transcription quantitative polymerase chain reaction (RT-qPCR) assays for cancer diagnosis and could be paired with a simple portable device for point-of-care clinical testing.
The research, led by Yong Zeng, Ph.D., an associate professor in the department of chemistry, an affiliate faculty member in the J. Crayton Pruitt Family department of biomedical engineering and a UF Health Cancer Center member, was published on April 27 in Nature Biomedical Engineering. The team collaborated with the department of surgery in the UF College of Medicine to collect the samples tested in the study.
The method works by detecting microRNAs, which are small RNA molecules involved in regulating gene expression, in tiny particles circulating in the blood called extracellular vesicles.
MicroRNAs have emerged as promising sources for developing biomarkers of cancerous tumors in human fluids, such as blood. But clinical application has remained limited because of the complexity of microRNAs and the lack of a tool sensitive enough to detect them.
Extracellular vesicles are nanosized particles, actively released by cells, that play important roles in cell functions and diseases by shuttling biomolecules among cells. Tumor cells seem to more aggressively produce the extracellular vesicles in microRNAs associated with disease, “making them an exciting source for exploring new cancer markers,” Zeng said.
Recently, interest has been surging in CRISPR technology, a powerful gene editing tool that was the subject of the 2020 Nobel Prize in Chemistry, as a way to develop new bioassays for disease diagnostics. But earlier CRISPR tests involved multistep reactions with manual handling and were not as sensitive as the gold standard, RT-qPCR.
“Our idea was to design a way to simplify the entire workflow into ‘one pot,'” Zeng said. “We designed a fast, sensitive method to detect microRNAs that is simpler and has a low risk of cross-contamination.”
The “one pot” method means that every chemical agent that is needed except for the sample is stored in one test tube. To perform the analysis, only the microRNA sample needs to be added to elicit a reaction.
“To our surprise, we found that our method has comparable sensitivity to PCR tests,” Zeng said. “Our method has excellent analytical and diagnostic performance while substantially expediting the workflow.”
The team focused on adapting the technology for pancreatic cancer given the high mortality of the disease. Pancreatic cancer is difficult to detect early. Often, the tumor has spread and cannot be completely removed by the time it’s diagnosed, preventing the chance of a cure.
“Compared to other types of cancers, diagnostic testing for pancreatic cancer has not changed significantly in nearly 50 years,” Zeng said. “There is significant interest in developing biomarkers for pancreatic cancer diagnosis.”
The method, called “EXTRA-CRISPR,” represents a game-changing method in the microRNA testing field, said He Yan, Ph.D., a postdoctoral researcher in Zeng’s lab and the paper’s first author.
“Our method is very promising for diagnosis of cancer, such as pancreatic cancer, when combined with robust microRNA biomarkers and for point-of-care testing,” he said. “In the future, this method can be coupled with a very simple, low-cost portable device to make pancreatic cancer detection simpler but still reliable.”
As a proof of concept, the team demonstrated that the new one-pot assay could be adapted for two detection methods commonly used for point-of-care testing. A portable smartphone-based device was first prototyped using off-the-shelf components, including a blue LED illuminator, a plastic filter and a coffee mug warmer. The team assembled these components on a 3D-printed body part. A smartphone was mounted on the device to obtain fluorescence images of the test vials after the reaction to measure the target markers.
In addition to the fluorescence detector, they paired the EXTRA-CRISPR assay with a commercially available lateral-flow test strip to build an instrument-free point-of-care device. The team tested both point-of-care methods for detecting pancreatic cancer, and they yielded diagnostic results consistent with those obtained with a benchtop PCR machine.
The researchers have filed a patent application based on the work to help make the one-pot CRISPR assay and the point-of-care technology broadly available for basic research and clinical use.
More information:
He Yan et al, A one-pot isothermal Cas12-based assay for the sensitive detection of microRNAs, Nature Biomedical Engineering (2023). DOI: 10.1038/s41551-023-01033-1
Journal information:
Nature Biomedical Engineering
Source: Read Full Article