Researchers connect Alzheimers-associated genetic variants with brain cell function
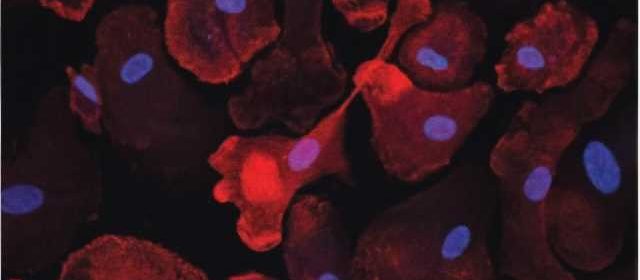
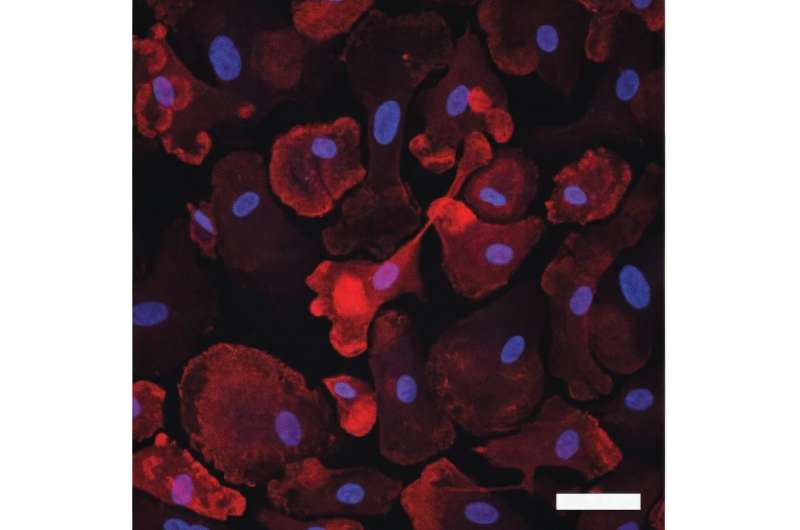
Scientists studying Alzheimer’s disease (AD) have identified thousands of genetic variants in the genome in the development of this progressive neurodegenerative disease.
These variants are predominantly located in genomic regions that do not code for proteins, making it difficult to understand which variants confer individuals’ risk of AD. Non-coding variants were once thought to be “junk DNA” by scientists.
In recent years, these variants have been appreciated for playing crucial roles in controlling gene expression across tissues and cell types. However, linking these non-coding variants to the genes they regulate and effects on AD-related functions is a daunting task.
Now, researchers at the University of North Carolina at Chapel Hill and The University of California, San Francisco, have identified the connections of risk variants with functions in microglia and then how they may contribute to AD.
“Microglia are brain’s immune cells and are critically important for AD,” said Yun Li, professor of genetics and biostatistics in the UNC School of Medicine and UNC Gillings School of Global Public Health. “Our study focuses squarely on the critical genomic regions that are important for regulating microglia cells. These variants and regions we’ve uncovered will serve as a great starting point for conducting further experiments in microglia.”
Li and Yin Shen, Ph.D., associate professor at the Institute of Human Genetics and the Department of Neurology at UC-San Francisco, and their teams performed a detailed analysis in microglia of potential functional regions harboring genetic variants associated with AD. They discovered 181 new regions of interest containing 308 prioritized variants, which were previously not considered to play a role in Alzheimer’s disease. Their results were published in Nature Genetics.
Fine-mapping and CRISPRi
Li and her colleagues started from 37 genetic loci associated with AD to prioritize risk variants and their residing potential functional regions—termed candidate cis-regulatory regions (cCRE)—in microglia, they performed a process called fine-mapping. One locus at a time, they studied the associated variants with a special consideration of epigenetic signatures and 3D genome interaction annotations indicating their likelihood of functioning in microglia.
After prioritizing variants that are most likely to exert their effect on AD through gene regulatory function in microglia, they performed CRISPR interference (CRISPRi) screening experiments to nail down the exact regions that affect microglia gene expression using human pluripotent stem cell differentiated microglia.
Using this epigenomic editing technology, the researchers can “perturb” candidate regions to see whether any tested genomic regions can impact downstream gene expression. They found that turning off one region can often impact a “whole neighborhood” of genes, much like a blackout on a power grid.
“We have been asking the wrong question,” said Li. “We should be asking what the targeting gene or genes of these variants are affecting the microglia. Sometimes, one variant may affect the expression of multiple genes in the neighborhood.”
Identifying one among the rest
Additionally, each region could contain several AD-associated genetic variants. Researchers then needed to pinpoint which variants are causal among the many that were identified through genetic analysis. Such precision is crucial for understanding the mechanisms by which non-coding variants contribute to the development of AD.
The team employed a cutting-edge genome editing technique—prime editing, which allows them to introduce one single DNA base substitution at a time and to assess individual variant function at the TSPAN14 AD risk locus. Through this method, they were able to identify one specific variant, differentiating it from another which is almost perfectly correlated and in the same cCRE region, to be responsible for TSPAN14 expression.
Linking non-coding variants to functions beyond gene expression
More importantly, the responsible variant further negatively affected a cascade of downstream cellular processes, including the maturation of ADAM10 protein and soluble TREM2 shredding in microglia. Since all three aforementioned genes are known to be risk genes for AD, the study successfully links an AD non-coding variant to functions in microglia beyond control of gene expression.
Their research findings, Li said, will serve as a new foundation from which other researchers can discover more causal variants of AD, predict disease risks, and develop more effective therapies. This work was also made possible in collaboration with Li Gan’s group from the Helen and Robert Appel Alzheimer’s Disease Research Institute, Weill Cornell Medical College.
Li, Shen, and Gan labs will continue to expand the analysis of AD risk variants using more complex model systems that mimic the human brain, such as human cerebral organoids.
More information:
Xiaoyu Yang et al, Functional characterization of Alzheimer’s disease genetic variants in microglia, Nature Genetics (2023). DOI: 10.1038/s41588-023-01506-8
Journal information:
Nature Genetics
Source: Read Full Article