New therapy helps immune system eradicate brain tumors
Researchers from Uppsala University have developed a method that helps immune cells exit from blood vessels into a tumor to kill cancer cells. The goal is to improve treatment of aggressive brain tumors. The study has been published in the journal Cancer Cell.
Glioblastoma is an aggressive brain tumor that lacks efficient treatment. This is in part due to the ability of the tumor to suppress or evade the body’s natural anti-cancer immune response. Immunotherapy, using checkpoint inhibitors, can reactivate the immune system against cancer. However, for this type of treatment to be effective, specific immune cells known as killer T cells must be present within the tumor.
Unfortunately, blood vessels in brain cancer are dysfunctional and act as a barrier, preventing killer T cells from reaching the tumor. As a result, this form of immunotherapy, which is effective against many forms of cancer, is ineffective against brain cancers.
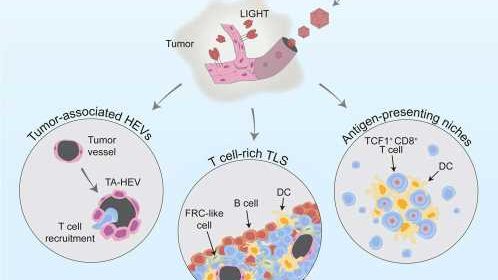
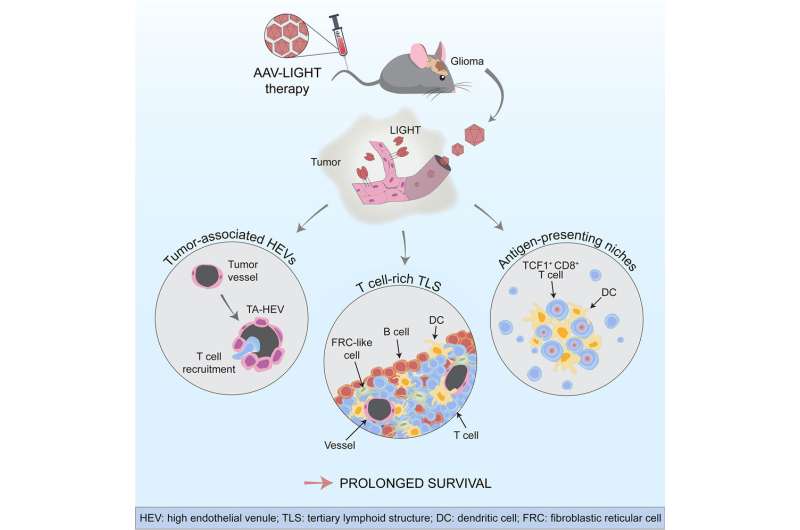
In the new study, the Uppsala researchers have developed a method to help the killer T cells reach the tumors and fight cancer cells. They used a viral vector that specifically infected the blood vessels in the brain and enabled them to produce a factor called LIGHT. This altered the function of the tumor vessels, increasing their ability to transport T cells from the blood into the tumor tissue.
“We found that the tumor blood vessels changed their shape and function when we used the viral vector AAV-LIGHT as a therapy in our experimental glioblastoma models. The viral vector induces production of LIGHT in the blood vessels, which tailors their function to recruit killer T cells into the tumor. This also creates a beneficial environment around the blood vessels which supports killer T cell function,” says Professor Anna Dimberg who led the study together with Professor Magnus Essand.
AAV-LIGHT also induced the formation of immune cell aggregates known as tertiary lymphoid structures (TLS) in association with the brain tumor. Such structures resemble lymph nodes and their presence within tumors has been associated with an increased sensitivity to cancer immunotherapy.
“It is exciting that TLS are formed when we use AAV-LIGHT therapy, since it is believed that killer T cells can be activated against the tumor cells in these structures. We also found that the treatment prolonged survival and in some cases was curative in our experimental models,” says researcher Mohanraj Ramachandran who shares first authorship of the study with doctoral students Alessandra Vaccaro and Tiarne van de Walle.
The researchers also found that AAV-LIGHT therapy skewed the composition of the TLS to include large numbers of T cells.
“We were intrigued to find out that we could manipulate TLS composition to our advantage using our therapy. This is new knowledge that could be used as a tool to alter the function of these structures in multiple settings,” says Vaccaro.
In addition, the new therapy promoted a special population of killer T cells, called “stem-like T cells,” that localized both within the TLS and in specialized niches that formed around the blood vessels in the tumor.
“We were excited to find that AAV-LIGHT treatment increased the presence of stem-like T cells, as they are known to boost the effects of immunotherapy. Finding them within TLS and niches tells us about the importance of the interplay between the various effects of AAV-LIGHT,” says Tiarne van de Walle.
The researchers now want to further develop this new therapy to determine if AAV-LIGHT can be used for patients with glioblastoma.
“The viral vector needs further development before we can initiate clinical studies, but the results are very promising. We hope that this new therapy will improve the chances for patients with glioblastoma in the future,” says Essand.
More information:
Mohanraj Ramachandran et al, Tailoring vascular phenotype through AAV therapy promotes anti-tumor immunity in glioma, Cancer Cell (2023). DOI: 10.1016/j.ccell.2023.04.010
Journal information:
Cancer Cell
Source: Read Full Article