New study offers hope for pulmonary fibrosis patients
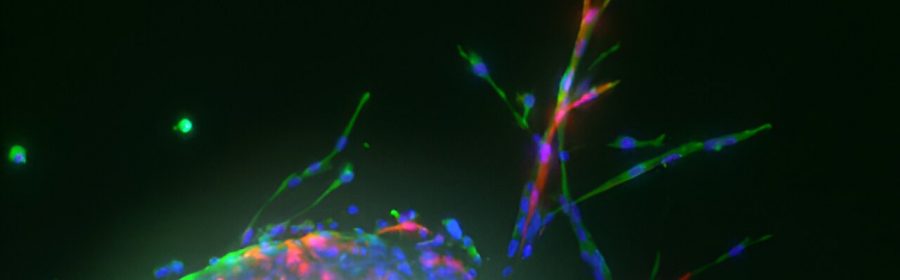
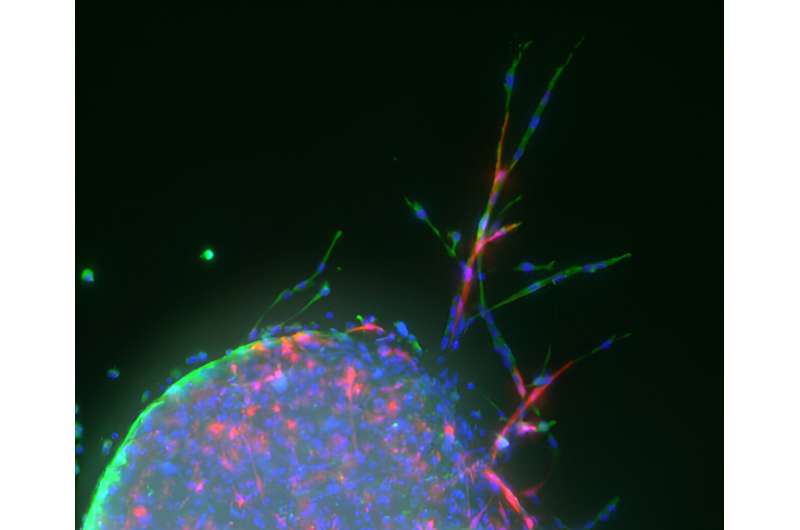
Using a new recipe for growing blood vessels from living lung tissue in the lab, a University of Virginia School of Engineering and Applied Science research team has developed an analytical tool that could lead to a cure for idiopathic pulmonary fibrosis, or IPF, a lung-destroying disease.
Fibrosis is chronic scarring of tissue and it can strike nearly every system in the body. According to the National Institutes of Health, the government estimates that 45% of deaths in the United States can be attributed to fibrotic disorders. In the lungs, fibrosis restricts breathing, so understanding how scarring occurs, and ultimately how to stop it, are essential questions—especially in the case of IPF, a form of pulmonary fibrosis with no known cause.
In search of answers, assistant professor of chemical engineering Lakeshia J. Taite is leading a team in collaboration with Shayn M. Peirce-Cottler and biomedical engineering Ph.D. student Julie Leonard-Duke in Peirce-Cottler’s lab. Peirce-Cottler is the chair of biomedical engineering and Harrison Distinguished Teaching Professor.
Their work combines computational models of how blood vessels behave in the fibrotic lung developed by Leonard-Duke in Peirce-Cottler’s lab with experiments using hydrogels engineered in Taite’s lab. The result is a new investigative platform for studying the formation of blood vessels—a process called angiogenesis.
Taite and Pierce-Cottler want to understand the role of angiogenesis—a natural part of tissue repair after injury—when the lungs won’t stop trying to heal, turning pliant tissue stiff and fibrous until they no longer function.
The research was published in Microcirculation, whose editors selected a figure from the paper to feature on the cover of the August 2023 issue.
The image shows vascular sprouting from mouse lung tissue planted on a hydrogel, a water-swollen biomaterial that resembles a soft contact lens. Taite’s lab functionalizes these hydrogels with bioactive molecules that closely mimic the angiogenic signals that encourage blood vessel development.
To achieve this functionality, Taite’s team chemically couples specific peptides—strings of amino acids, the building blocks of proteins—with derivatives of polyethylene glycol, a common off-the-shelf crystalline polymer, to form a PEG-peptide conjugate. The peptides are either purchased or made using a programmable synthesizer.
This multistep process produces a fluffy white powder akin to store-bought gelatin mix. Dissolved in a water-based solution and exposed to ultraviolet light, the molecules crosslink to form a soft but solid material that mouse lung cells planted on the surface can interact with, triggering new cell growth.
“The hydrogel was tailored to have mechanical properties—for example, stiffness and elasticity—matching healthy lung tissue,” Taite said. “The hydrogel acts as the vascular cells’ native extracellular matrix, the complex mixture of proteins, carbohydrates and minerals that provide important cues for tissue development and maintenance.”
The broad goal of the project is to understand the biomechanical and biochemical cues to blood vessels in the lungs during the development and progression of fibrosis. The team is building laboratory modeling systems to accelerate the search for treatments to stop IPF in its tracks.
“This project represents a novel angiogenesis assay that allows investigation of matrix stiffness on microvascular sprouting,” Taite said.
Taite began the research just after her arrival at UVA in 2021, using her experimental techniques with Leonard-Duke’s computer models to validate and improve the models, which in turn inform Taite’s experiments.
“We incorporate data from this sprouting assay into our computer models which simulate the complex cell behaviors contributing to lung fibrosis,” Leonard-Duke said. “We then use artificial intelligence and machine learning approaches to comprehensively explore the genes and proteins that could be targets for new drugs.”
Also contributing to the research were Anthony C. Bruce, biomedical engineering lab manager and a co-author of the paper, Samuel Agro, a now second-year chemical engineering Ph.D. student in Taite’s lab, and Yixuan Yuan, who graduated with his B.S. in chemical engineering in spring 2023.
Agro and Leonard-Duke are working on a continuation of the project.
The paper “Variations in mechanical stiffness alter microvascular sprouting and stability in a PEG hydrogel model of idiopathic pulmonary fibrosis” is published in Microcirculation.
More information:
Julie Leonard‐Duke et al, Variations in mechanical stiffness alter microvascular sprouting and stability in a PEG hydrogel model of idiopathic pulmonary fibrosis, Microcirculation (2023). DOI: 10.1111/micc.12817
Journal information:
Microcirculation
Source: Read Full Article