MTAP loss in metastatic breast cancer patients: Genomic landscape
A new research paper titled “Genomic landscape of metastatic breast cancer (MBC) patients with methylthioadenosine phosphorylase (MTAP) loss” has been published in Oncotarget.
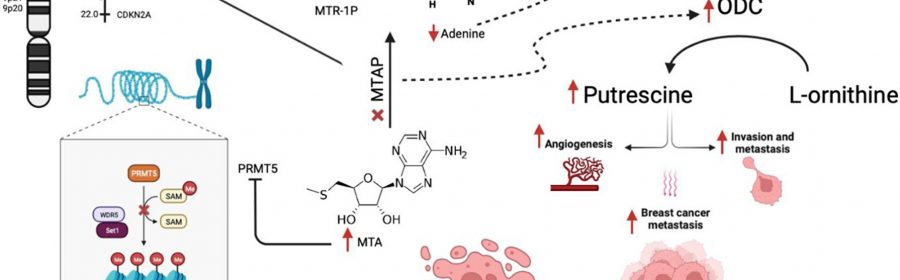
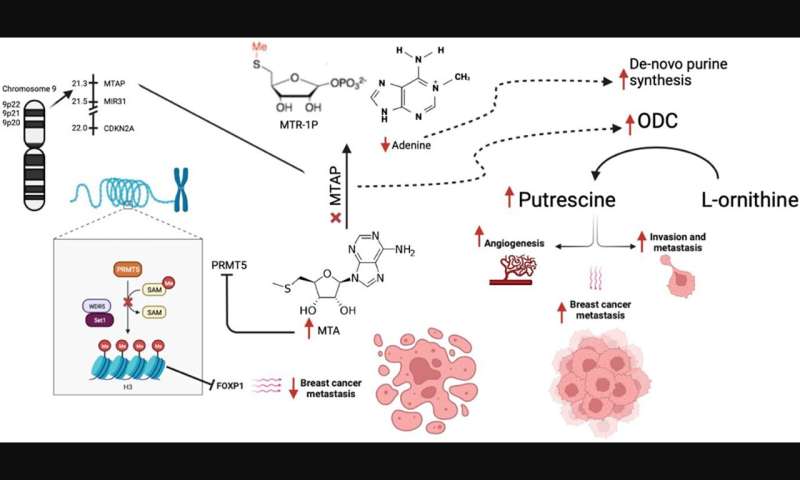
Homozygous deletion of methylthioadenosine phosphorylase (MTAP) upregulates de novo synthesis of purine (DNSP) and increases the proliferation of neoplastic cells. This increases the sensitivity of breast cancer cells to DNSP inhibitors such as methotrexate, L-alanosine and pemetrexed. In their recent study, Maroun Bou Zerdan, Prashanth Ashok Kumar, Elio Haroun, Nimisha Srivastava, Jeffrey Ross, and Abirami Sivapiragasam from SUNY Upstate Medical University and Foundation Medicine, Inc. analyzed 7,301 metastatic breast cancer (MBC) patients who had undergone hybrid-capture based comprehensive genomic profiling (CGP).
“We provide one of the first large analyses of the spectrum of GA [genomic alterations] occurring in MTAP deleted MBC with the hope that this would enable identifying potential therapeutic agents in the future,” the researchers explain.
Tumor mutational burden (TMB) was determined on up to 1.1 Mb of sequenced DNA and microsatellite instability (MSI) was determined on 114 loci. Tumor cell PD-L1 expression was determined by IHC (Dako 22C3). 208 (2.84%) of MBC featured MTAP loss. MTAP loss patients were younger (p = 0.002) and were more frequently ER− (30% vs. 50%; p < 0.0001), triple negative (TNBC) (47% vs. 27%; p < 0.0001) and less frequently HER2+ (2% vs. 8%; p = 0.0001) than MTAP intact MBC.
Lobular histology and CDH1 mutations were more frequent in MTAP intact (14%) than MTAP loss MBC (p < 0.0001). CDKN2A (100%) and CDKN2B (97%) loss (9p21 co-deletion) were significantly associated with MTAP loss (p < 0.0001). Likely associated with the increased TNBC cases, BRCA1 mutation was also more frequent in MTAP loss MBC (10% vs. 4%; p 20 mut/Mb levels in the MTAP intact MBC (p < 0.0001) and higher PD-L1 low expression (1–49% TPS) in the MTAP loss MTAP (p = 0.002) were observed.
The researchers conclude, “MTAP loss in MBC has distinct clinical features with genomic alterations (GA) affecting both targeted and immunotherapies. Further efforts are necessary to identify alternative means of targeting PRMT5 and MTA2 in MTAP-ve cancers to benefit from the high-MTA environment of MTAP-deficient cancers.”
More information:
Maroun Bou Zerdan et al, Genomic landscape of metastatic breast cancer (MBC) patients with methylthioadenosine phosphorylase (MTAP) loss, Oncotarget (2023). DOI: 10.18632/oncotarget.28376
Journal information:
Oncotarget
Source: Read Full Article