Insight into the Different Clinical Trial Study Designs

Clinical trials are well-controlled and designed experiments that aim to prove that an Investigational Medicinal Product (IMP) is effective. Clinical trial data is submitted to the national health authority which then determines if the IMP or drug is effective. Therefore the types of studies carried out are crucial to proving the efficacy of the drug and obtaining approval. This article aims to provide an overview of different clinical study designs and their use in clinical trials.
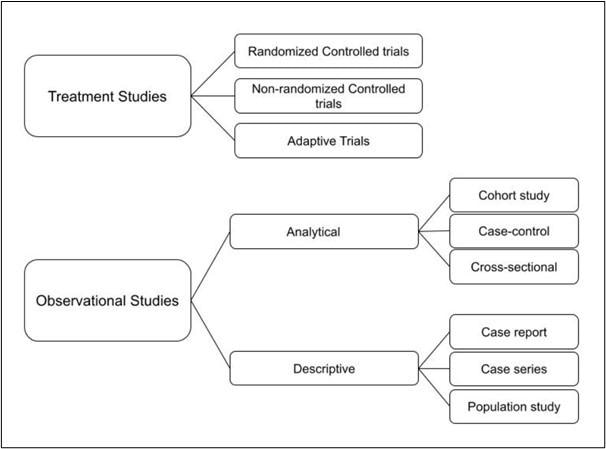
Fig 1. Diagram showing the main types of clinical study designs
Treatment Studies
Treatment studies aim to analyze the effect of an intervention such as a drug on a group of people. These types of studies are most commonly used in clinical trials and the 3 main types of treatment studies are:
- Randomized controlled trials (RCT) is a rigorous tool to analyze cause-effect relationships between an intervention and an outcome. Participants are selected based on eligibility criteria and are then randomly assigned to 2 or more groups to test the effectiveness or other factors of a drug, treatment, or other intervention. RCT often entails analyzing the response of a group that receives an intervention against a control group that receives a placebo; the differences between the two groups are recorded and analyzed.
As the participants are randomly assigned to the group, bias is reduced and participant characteristics are balanced meaning that any differences between the two groups are attributed to the intervention.
The subcategory of RCT is a blind trial or non-blind trial; this refers to whether information that might influence the results of the trial is withheld. This could be the allocation of the group that receives the intervention such as the IMP or the placebo. This information may be withheld from participants and researchers to further minimize bias, this is known as a randomized double-blinded trial and is the gold standard of trials. These are highly reliable trials; however, the drawbacks include high cost and labor-intensive.
- Non-randomized controlled trials are used when RCT is unethical or not possible such as in surgical interventions where consent for randomization is difficult to obtain and difficult to conceal from the patient and surgeon. Therefore, non-randomized controlled trials are the alternative to study and cause-effect relationships. The experimenter determines which participants are assigned to an intervention group or not; there are usually qualifiers and criteria that determine which participants are assigned to the intervention group.
This type of experiment is also useful as "pre-post testing". Data on the participants are gathered before the intervention and recorded to analyze if any participants have certain tendencies that might influence the result of the trial.
- Adaptive clinical trials are used to evaluate a drug or medical device by observing the outcomes such as effectiveness or side effects. This type of design allows the researchers to make modifications during the trial such as increasing the dose or duration based on the participant outcome. This adaptation is continued throughout the trial as specified by the protocol. The main advantage is that it allows for faster results as researchers can respond to the data in real-time and focus on the patient population or dosages that are observed to be effective. Traditionally, a clinical trial would follow a predetermined plan regardless if there was a specific result that seemed more effective, and once completed the results would be analyzed and decisions of efficacy made. Adaptive trials have allowed experimental cancer drugs to rapidly deliver promising results that wouldn't be possible with traditional study designs.

Image Credit: Irina Strelnikova/Shutterstock.com
Observational Studies
These are rarely used in clinical trials of a drug and mostly aims to study people in different contexts without intervention. There are two subcategories in observational studies:
Descriptive study
This research design aims to obtain information to systematically describe a situation, population, or phenomenon. It doesn’t aim to quantify a relationship or examine the reason behind it. They are not considered very reliable and are rarely used in clinical trials. Descriptive studies are mainly used in post-surveillance stages after clinical trials and a drug has been approved.
The different types include:
- Case reports provide detailed information on a particular case and are an anecdotal form of evidence; they are at the bottom of the hierarchy of clinical evidence. The adverse effect of thalidomide was identified through a series of case reports.
- Case series track patients that have a known exposure to certain medications and observe them over time; this can be used in phase II clinical trials.
- Population studies are the study of a group of people with a common characteristic such as race and observing their response, for example, to a specific treatment.
Analytical studies:
In Phase IV of clinical trials, pharmacovigilance studies after the approval of the drug may require observational studies such as cohort studies or case-control studies.
- Cohort studies observe a group of people with a defining feature or characteristic and study them at intervals through time – performing a cross-section at these intervals. They are mainly used to refute or prove a suspected association between a cause and effect; such as the effectiveness or side effects of a specific drug after approval.
- Case-Control studies identify groups of people with different outcomes and compare them to identify factors that led to the outcome. The participants are not randomly assigned to different groups but are observed to determine their outcome and cause.
- Cross-sectional studies that analyze data on a population, rather than an individual, at a specific point in time are carried out at a specific point in time. The aim is to provide data on the risk and prevalence of a certain disease in a population rather than provide cause and effect.
Summary
There are many different types of clinical trial study designs and the main types used are treatment studies as they are most reliable due to their rigorous control of different variables that ensure any change in outcome is due to the intervention or IMP. Observational studies are rarely directly used in clinical trials but it is used in pharmacovigilance studies after a drug is approved.
References:
- Hariton, E., & Locascio, J. J. (2018). Randomised controlled trials – the gold standard for effectiveness research: Study design: randomised controlled trials. BJOG : an international journal of obstetrics and gynaecology, 125(13), 1716. https://doi.org/10.1111/1471-0528.15199
- Cancer Research UK. (2021, December 10). Types of clinical trials. https://www.cancerresearchuk.org/about-cancer/find-a-clinical-trial/what-clinical-trials-are/types-of-clinical-trials
- Axelrod D.A., Hayward R. (2006) Nonrandomized Interventional Study Designs (Quasi-Experimental Designs). In: Penson D.F., Wei J.T. (eds) Clinical Research Methods for Surgeons. Humana Press. https://doi.org/10.1007/978-1-59745-230-4_4
- Bakhai, A., & Wang, D. (Eds.). (2004). Clinical trials : A practical guide to design, analysis, and reporting. Remedica Medical Education and Publishing.
Further Reading
- All Clinical Trial Content
- What is a Phase 0 Clinical Trial?
- What is a Phase 1 Clinical Trial?
- What is a Phase 2 Clinical Trial?
- What is a Phase 3 Clinical Trial?
Last Updated: Mar 31, 2022

Written by
Storay Amiri
Storay attended the University College of London (UCL) to Study Master of Pharmacy and graduated in 2021 with First Class Honors. A four-year integrated master degree with a detailed focus on all parts of medication, from chemistry to body therapeutics, pharmacology, pharmaceutics, and more.
Source: Read Full Article