Boston partnership leverages local manufacturing to quickly produce reusable face shields


Researchers at a major Boston academic medical center designed, fabricated, tested, and implemented a reusable face shield for front-line medical staff within a couple of weeks. The work, presented June 18 in the journal Med, was carried out at the Brigham and Women’s Hospital Emergency Department in collaboration with members of the volunteer group Greater Boston Pandemic Fabrication Team (PanFab) and the local maker community.
The authors of the study say it provides an efficient and generally applicable approach for leveraging local manufacturing processes to quickly produce personal protective equipment (PPE) during public health emergencies. The paper provides all of the instructions and model files to produce the face shields.
“All of the designs and protocols generated through this effort are being freely shared for reuse and improvement, and the results of our testing in the emergency department at the Brigham are reported in full to facilitate the execution of similar face-shield efforts in other clinical settings,” says co-senior study author Sherry Yu of the Brigham and Harvard Medical School. “We anticipate that this work will provide a framework for the design and implementation of similar approaches to PPE manufacturing for current and future shortages.”
In the face of a rapidly expanding coronavirus disease 2019 (COVID-19) pandemic, severe PPE shortages have put both health care professionals and patients at increased risk of infection. The origins of these shortages reflect the fragility of medical supply chains, in which relatively few international vendors dominate critical medical product areas. Because many hospitals use just-in-time inventory management, supply-chain problems rapidly deplete hospital supplies and prevent restocking from traditional vendors. Two months into the COVID-19 pandemic in the US, traditional supply chains remain substantially disrupted, and much-advertised alternative sources of supply from large companies have not yet been widely distributed.
Faced with this crisis, many caregivers and medical centers have turned to local fabricators that use 3-D printing to provide replacements for products such as face shields, filtering respirators, and even ventilator components. Recently, many public-domain designs for face shields have become available. Groups using these designs have found that introducing face shields and similar PPE into a healthcare setting is challenging. Despite their relatively simple design, face shields are regulated in the U.S. by the Centers for Disease Control and the Food and Drug Administration (FDA). In April, the FDA issued a letter stating that it “does not intend to object to individuals’ distribution and use of improvised PPE when no alternatives, such as FDA-cleared masks or respirators, are available.”
Still, no clear path exists for introducing a locally fabricated product into a clinical setting. “The FDA letter provides a legal framework in which to use locally fabricated, improvised PPE in the US, but it does not address the more general issue of introducing non-traditional PPE into a healthcare environment; what to do if emergency guidance is lacking, as it currently is in many countries; and how nontraditional devices should be introduced into the hospital supply chain in a rational, safe and controlled manner,” says co-senior study author Nicole LeBoeuf of the Brigham and Harvard Medical School.
To address this need, Yu, LeBoeuf, and co-senior study author Peter Sorger of Harvard Medical School used a research protocol under supervision from the university’s institutional review board to test a locally fabricated face shield as an investigational device. This allowed clinicians to participate in an iterative design process that was followed by real-world testing in an emergency department. Yu says this approach is unique because feedback was obtained in parallel, leading to faster implementation.
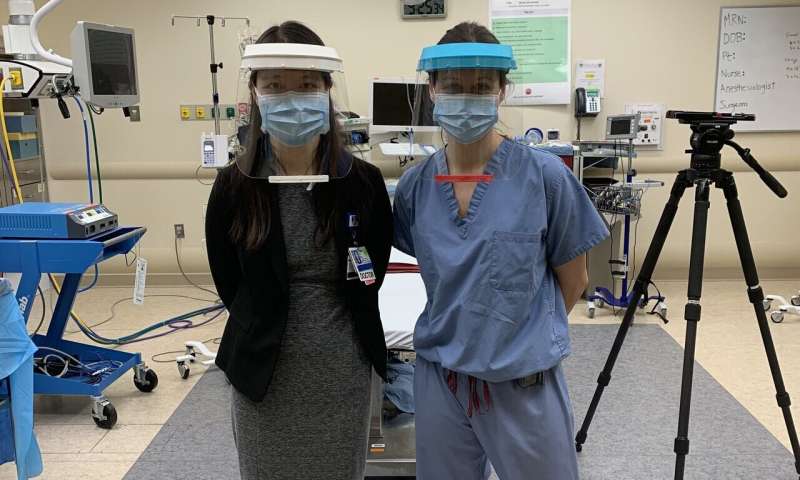
The researchers started with the 3-D-printed Prusa RC2 face-shield design developed in the Czech Republic. They improved on this design to ensure limited aerosol and splatter exposure coming from the front and above, resistance to fogging, and suitable comfort for all-day wear in a high-intensity clinical setting. Overall, four substantial design modifications were made on the basis of clinical feedback, and the resulting design includes many features that are not present in disposable commercial face shields.
First, the researchers increased the width to maximize face protection without obstructing hearing or impeding a user’s range of motion. Second, they used hook-and-loop Velcro to adapt each visor to individual users. Third, they reduced tightness by placing anchor points for the hook-and-loop strap in-line with the headbands.
And fourth, the researchers added a visor above the headband to prevent fluid from entering the top of the face shield during high-risk procedures in which a clinician is required to lean forward. This includes endotracheal intubation for mechanical ventilation, one of the riskier procedures that must be performed on COVID-19 patients. They also added a plastic lip at the bottom of the visor so that any liquid that did fall on this area would be retained by the lip and would not spread down the front of the shield and affect a user’s vision.
Among 92 surveyed staff members of the emergency department, most responded that the BWH/PanFab face shield was better in terms of splash protection (96%), sturdiness and reliability (92%), ease of use (78%), and comfort (87%). Moreover, 92% planned to continue using the BWH/PanFab face shield, and 99% responded they would be comfortable or very comfortable using this shield in a clinical scenario in which they had no other option.
The entire process of introducing a non-traditionally manufactured product into a hospital supply chain in a safe and controlled manner took only three weeks. With a design in hand and institutional support, others can deploy this process in two weeks or less, plus two to three days for clinical testing if design modifications are necessary because of material shortages. To date, the researchers have fabricated approximately 3,000 face shields, all of which remain in use. Currently, they are assessing various procedures for sterilizing and reusing face shields and similar PPE and clinically testing an alternative face-shield design that can be packed flat to facilitate shipping and storage.
Sorger says the changes in regulatory guidance are needed to facilitate the type of testing they used for their face shields. Moreover, hospital systems need staff their crisis and response teams with individuals who are specifically tasked with outreach to maker and manufacturing communities.
Source: Read Full Article