Adult neurogenesis may hold clues for more effective treatment of alcoholism

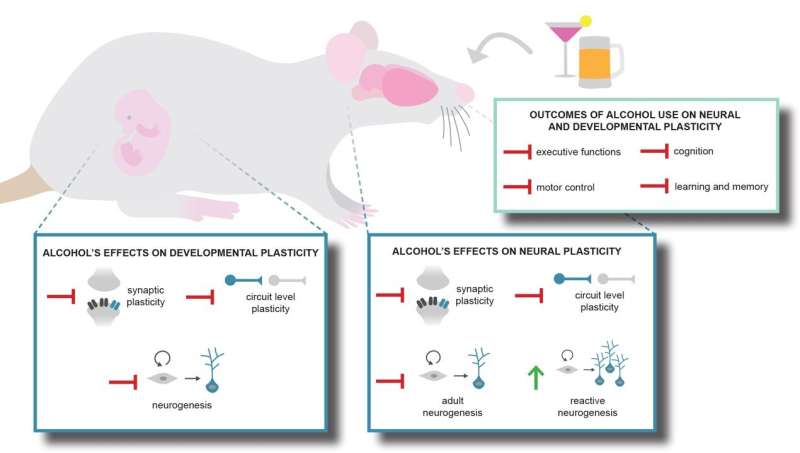
Neuroplasticity, the remarkable ability of the brain to modify and reorganize itself, is affected by or in response to excessive alcohol, whether through individual consumption or exposure in the womb. It is now well accepted that the birth and integration of new neurons continue beyond development and into adulthood. New discoveries and insights on how alcohol impacts this and other plastic processes are discussed in “Alcohol and Neural Plasticity,” a special issue of Brain Plasticity.
“The discovery and evolution of our acceptance of the role of adult neurogenesis in brain structure and function have revolutionized our understanding of the brain’s response to insult, but has also introduced a potential mechanism of recovery in some regions,” explains Guest Editor Kimberly Nixon, Ph.D., The University of Texas at Austin, College of Pharmacy, Austin, TX.
In models of Fetal Alcohol Spectrum Disorder, earlier research found that gestational exposure to moderate levels of alcohol in mice throughout a period equivalent to the first and second human trimesters profoundly impacted neurogenesis. In a follow up study published in this special issue, lead investigator Lee Anna Cunningham, Ph.D., Department of Neurosciences, University of New Mexico School of Medicine, Albuquerque, NM, USA, and colleagues examined the functional and structural consequences of prenatal alcohol exposure on adult-generated neurons. They found no direct effects of prenatal alcohol exposure on adult hippocampal neurogenesis in mice housed under standard conditions, but prenatal alcohol exposure impaired the neurogenic response to enriched environment. These mice also performed poorly in a neurogenesis-dependent pattern discrimination task and displayed impaired enrichment-mediated increases in dendrite complexity.
“This study further underscores the impact of moderate gestational alcohol exposure on adult hippocampal plasticity and supports adult hippocampal neurogenesis as a potential therapeutic target to remediate certain neurological outcomes in fetal alcohol syndrome,” notes Dr. Cunningham.
The mechanisms of recovery from adult alcohol use disorder are not clear, although reactive neurogenesis has been observed following alcohol dependence. Dr. Nixon and colleagues studied the role of adult-born neurons in the recovery of hippocampal learning and memory during withdrawal and abstinence from alcohol dependence. They hypothesized that reducing reactive neurogenesis would impair functional recovery. Adult male rats were subjected to a four-day binge alcohol exposure, and then reactive neurogenesis was chemically inhibited. Despite reducing this potential mechanism of hippocampal repair, learning and memory behavior still recovered and were identical to controls.
“Further work is needed to better characterize and differentiate how adult-born neurons contribute to both hippocampal impairments in alcohol misuse but also recovery in abstinence,” Dr. Nixon says.
The special issue also reviews several key issues: the effect of combined alcohol and cocaine exposure on neural stem cells and adult neurogenesis; the neurotoxic effects of binge alcohol consumption, highlighting the scarcity of work on females and the aged; the role of immune activation as a mechanism of alcohol’s effects on synaptic and structural plasticity; and one of the first in-depth discussions of alcohol’s neurophysiological effects on hippocampal excitatory activity during alcohol withdrawal. This activity may underlie the hyperexcitability that is seen in alcohol withdrawal and can be a fatal complication of non-medically supervised “detox” from alcohol.
Also included are a review and data paper on alcohol effects on synaptic mechanisms that underlie the various behavioral deficits that occur with the development of alcohol abuse disorder and a developmental study that offers insight into our understanding of alcohol’s effects at synapses during juvenile development.
Source: Read Full Article