A hazard in breastfeeding: Perfluoroalkane acids, a persistent organic pollutant binding to human milk

Biomonitoring for polychlorinated dibenzodioxins and dibenzofurans (PCDD/PCDF) and polychlorinated biphenyls (PCB) has long traditions and was continued with the global monitoring plan (GMP) under the Stockholm Convention on Persistent Organic Pollutants.
The aim of the GMP is to apply one framework for sampling and analysis of so-called core matrices to detect temporal and spatial changes of POP concentrations. Due to inherent persistence and bioaccumulation of chlorinated POPs, the biomonitoring samples should be collected from primiparae, i.e., mothers having their first child, only.
This requirement could reduce the influences of individual factors from the donor mother and the chemical. Protocols to harmonize the identification, collection, and chemical analysis of the POPs in human milk had been developed and were updated periodically to incorporate newly listed POPs including the brominated flame retardants and perfluoroalkane substances (PFAS).
Perfluorooctane sulfonic acid (PFOS), its salts and perfluorooctane sulfonyl fluoride (PFOSF) was listed in 2009, amended in 2019, perfluorooctanoic acid (PFOA), its salts and PFOA-related compounds was listed in 2019. They have different physical and chemical properties than the initial chlorinated POPs and the later added polybrominated POPs (polybrominated diphenyl ether, hexabromocyclododecane, hexabromobiphenyl). They bind preferentially to proteins in the plasma.
Nevertheless, human milk remained the preferred matrix in the GMP and an aliquot from the national samples collected for the analysis of brominated/chlorinated POPs was recommended to be used for the analysis of the fluorinated POPs. Perfluoroalkane substances (PFAS) are a group of persistent substances that is suspected to cause several negative health effects including reduced birth weight, late puberty and lowered semen quality.
In humans, often levels of perfluorocarboxylic acids (PFCA) and perfluorosulfonic acids (PFSA) but also fluorotelomer compounds or replacements such as ammonium 4,8-dioxa-3H-perfluorononanoate, the ammonium salt of hexafluoropropylene oxide dimer acid (HFPO-DA) fluoride or 6:2 chlorinated polyfluorinated ether sulfonate are monitored at global level. Other PFAS, like perfluorohexanesulfonic acid (PFHxS) and perfluorononanoic acid (PFNA), had quite high detection frequencies but at lower concentrations.
Biomonitoring data using human milk are less than for blood, serum or plasma but have been reported from primiparae and multiparae. Most human milk studies found that concentrations for all PFAA analyzed were much lower than those in human blood. In all studies, PFOS and PFOA had the highest detection frequencies with variable results of recent researches.
With expanding the previous publication containing the data for PFOS, PFOA, and PFHxS from 44 human milk samples, the researchers from Örebro University of Sweden and Instituto Nacional de Controle de Qualidade em Saúde of Brazil have added other PFCA and PFSA which are not included in the list of recommended POPs in the GMP guidance document.
The results from historic stored samples from Brazil were also presented and discussed. This study entitled “Perfluoroalkane acids in human milk under the global monitoring plan of the Stockholm Convention on Persistent Organic Pollutants (2008–2019)” is published in Frontiers of Environmental Science & Engineering.
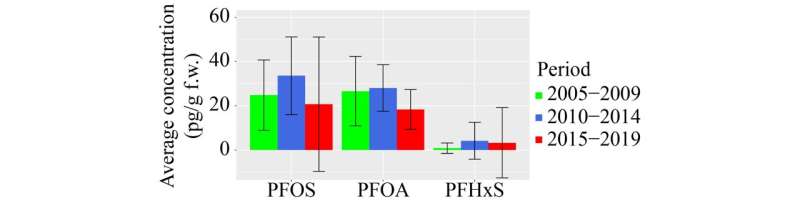
In 101 samples consisting of 86 national pools and 15 pools from States in Brazil obtained between 2008 and 2019, PFHxS was detected in 17% of the national pools and none in Brazil. PFOA and PFOS had a detection frequency of 100% and 92%, respectively. Other perfluoroalkane substances (PFAS) had either low detection frequencies and median values of zero (carboxylic acids C4–C11; except PFOA) or could not be quantified in any sample (sulfonic acids, C4–C10, and long-chain carboxylic acids, C12–C14).
Correlation between PFOA and PFOS was moderately (r = 0.58). Whereas median values were almost identical (18.9 pg/g f.w. for PFOS; 18.6 pg/g f.w. for PFOA), PFOS showed larger ranges (< 6.2 pg/g f.w.–212 pg/g f.w.) than PFOA (< 6.2 pg/g f.w.–63.4 pg/g f.w.). It was shown that wealthier countries had higher PFOA concentrations than poorer countries.
No difference in concentrations was found for samples collected in countries having or not having ratified the Stockholm Convention amendments to list PFOS or PFOA. The goal to achieve 50% decrease in concentrations within ten years was met by Antigua and Barbuda, Kenya, and Nigeria for PFOS and by Antigua and Barbuda for PFOA. In a few cases, increases were observed; one country for PFOS, four countries for PFOA.
With this study, no spatial and temporal trends could be established mainly due to uneven representation of countries from the UN regions and relatively short time periods between the measurements. Compared with PFOS, PFOA was more difficult to achieve the Stockholm Convention goal of 50% reduction in ten years.
Although some impact from geographic location (UN region) and lifestyle factors (income) were found, none of these seems to be a good indicator for PFOS and PFOA body burden.
Source: Read Full Article