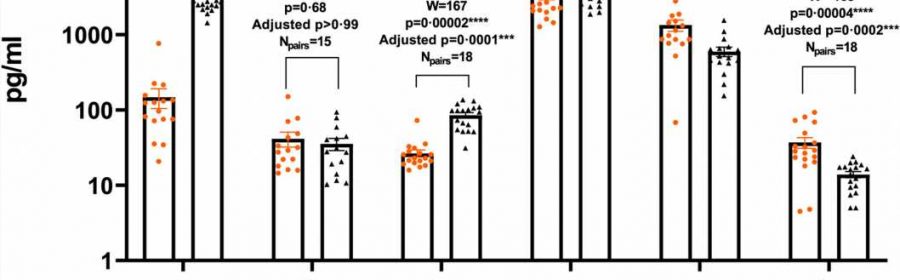
The exotic fruit shown to protect against vision loss

Eye health: Nutritionist reveals foods that protect your eyes We use your sign-up to provide content in ways you’ve consented to and to improve our understanding of you. This may include adverts from us and 3rd parties based on our understanding. You can unsubscribe at any time. More info Taking care of our eyes is vital, especially as we get […]
Continue reading »