How Does the Suprachiasmatic Nucleus (SCN) Control Circadian Rhythm?

Circadian rhythms are biological cycles within organisms that allow them to adjust their physiology and behavior to anticipate and adapt to changes in the outside environment. Circadian rhythms are maintained with the help of circadian clocks, the main circadian clock in mammals is the suprachiasmatic nucleus (SCN).
Neuroanatomy of the Suprachiasmatic Nucleus (SCN)
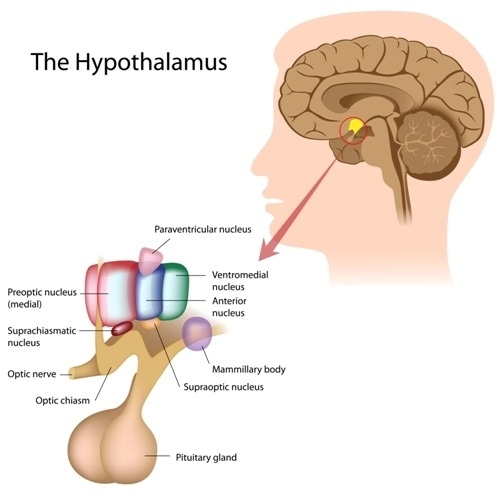
The SCN is located in the anterior region of the hypothalamus, and contains roughly 20,000 neurons. The SCN can be divided into two main sections depending on the neuropeptide expression.
The ‘Core’ of the SCN is mainly comprised of vasoactive intestinal peptide (VIP) expressing cells. The core mainly receives input from the retina and other brain regions.
On the other hand, the ‘Shell’ in mainly comprised of arginine vasopressin (AVP) expressing cells. The shell receives inputs mainly from the cortex, basal forebrain, and hypothalamus. The SCN sends outputs to various parts of the brain such as the medial areas of the hypothalamus and thalamus.
Inputs to the SCN
The SCN receives two types of input: photic and non-photic. The photic input comes from intrinsically photosensitive retinal ganglion cells (ipRGCs), which project through the retino-thalamic tract via glutamatergic synapses to neurons in the SCN. This helps to synchronise the circadian clock.
Exposure to light can alter the circadian rhythm, which is referred to as a 'phase shift'. Phase shifts can disrupt the normal responses to the circadian rhythm; for example, exposure to light during the night will affect sleep patterns via a phase shift.
The non-photic input to the SCN comes from other regions of the brain and helps to modulate the circadian rhythm. The SCN contain various serotonin (5-HT) receptors. 5-HT input from the midbrain raphe helps to modulate the SCN response to light by regulating phase shifts.
The intergeniculate nucleus (IGL) contains neuropeptide Y (NPY) expressing neurons, as well as gamma aminobutyric acid (GABA) expressing neurons. The IGL projects to the SCN via the geniculo-hypothalamic track, and induces phase shifts during the day. Stimulation of the medial raphe nucleus and the dorsal raphe nucleus increases the serotonin content in the SCN and IGL, respectively.
SCN Generation and Control of the Circadian Rhythm
The circadian rhythm generated by the SCN relies on delayed negative feedback in a core transcriptional feedback loop. CLOCK/BMAL1 dimers act at E-box promoter regions in the chromosome to promote transcription of various regulators of circadian rhythm (clock genes), such as various Period (PER)and Cryptochrome (CRY) genes. This results in an increase of PER and CRY proteins.
After a delay the PER/CRY dimers build up and start to inhibit transcription of their own genes. PER and CRY are also degraded by ubiquitin ligase complexes. These changes result in the decrease of PER and CRY, consequently lowering the inhibition of their transcription so a new cycle will eventually begin.
PER is simultaneously involved in a positive feedback loop, in which REV-ERBα acts at RORE promoter regions to inhibit BMAL transcription. PER binds to REV-ERBα which allows BMAL to be transcribed, thus allowing for more PER and CRY to be transcribed.
The E-box promoter region is also responsible for the transcription of clock-control genes (CCG) and the feedback loops discussed are responsible for the 24-hour cycle for the CCG expression. CCGs control various aspects of homeostasis and the cell cycle.
All neurons within the SCN oscillate with different phases, the cells combine outputs to give a rhythm, which is known as the SCN multi-oscillator network. VIP cells control light-induced phase resetting in the SCN and provide a coupling signal for SCN oscillators. This helps to stabilise and synchronise rhythms between individual SCN neurons. GABA signalling also helps to synchronise individual SCN neurons.
Effects of the Circadian Rhythm on the Body
The circadian rhythm is conveyed from the SCN to other parts of the brain. These downstream signals act on either the neuro-endocrine system or the pre-autonomic motor neurons of the hypothalamus, allowing for a multitude of physiological reactions to occur.
The SCN projects to the pineal gland to affect melatonin secretion, which can be achieved by the actions of VIP that activate adenyl cyclase. This increases the concentration of cAMP, which stimulates N-acetyltransferase, increasing the rate of melatonin synthesis.
The release of melatonin is the highest during the night and contributes to the sleep/wake cycle by preventing phase shifts, reducing sleep onset latency, increasing total sleep time, and inhibiting circadian arousal.
Cortisol is secreted from the adrenal gland and exhibits a circadian rhythm. Cortisol levels are lowest during the night and peak during the morning. Cortisol release is regulated by the hypothalamo-pituitary-adrenal axis (HPA).
The HPA receives NPY input from the SCN, which results in the release of adrenocorticotropic hormones from the corticotroph cells in the anterior pituitary gland, because of this cortisol is released from the adrenal gland. Cortisol has a negative feedback loop with the pituitary gland, in which rising levels of cortisol act to inhibit its secretion.
The circadian rhythm can also act on many other aspects of human physiology – including metabolism, body temperature and various elements of the immune system.
In Control of Physiological Processes
In conclusion, the SCN controls many physiological processes with the rhythm is generates. The transcription of clock genes in response to the day/night cycle are key to all processes affected by the circadian rhythm. The integration of photic and non-photic inputs to the SCN drive the cycle for the rhythm.
More research into the SCN and circadian rhythms will provide better and more detailed understanding of how the SCN generates the circadian rhythm and how other parts of the brain influences this. More research will also reveal additional information regarding how the circadian rhythm effects other parts of the brain in its downstream signalling.
Sources
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1994933/
- www.cell.com/…/0896-6273(95)90214-7.pdf
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5374060/
- https://link.springer.com/book/10.1007/3-540-27789-7
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4831275/?report=reader
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3475279/
- www.cell.com/…/S1550-4131(06)00241-5
Further Reading
- All Circadian Rhythm Content
- Circadian Rhythm
- Circadian Rhythm and Weight Loss
- Artificial Light Exposure and Circadian Rhythm
- Circadian rhythm length variations – early birds and night owls
Last Updated: Feb 26, 2019
Written by
Samuel Mckenzie
Sam graduated from the University of Manchester with a B.Sc. (Hons) in Biomedical Sciences. He has experience in a wide range of life science topics, including; Biochemistry, Molecular Biology, Anatomy and Physiology, Developmental Biology, Cell Biology, Immunology, Neurology and Genetics.
Source: Read Full Article