walls drug store

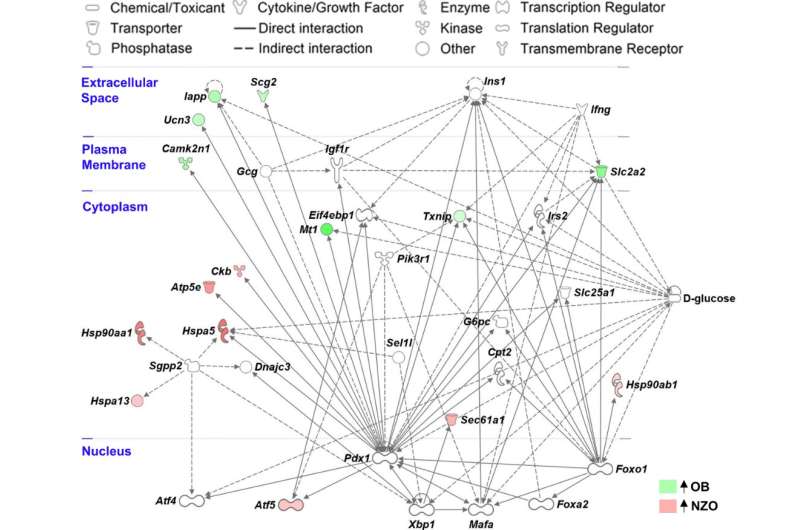
Progressive dysfunction and failure of insulin-releasing β-cells is a hallmark of type 2 diabetes (T2D). DZD researchers have now shown that diabetes-resistant and diabetes-susceptible mice respond differently to a carbohydrate-rich diet. The gene expression of the beta cells of the diabetes-resistant mice changed in such a way that a protective beta cell cluster developed. In diabetic-prone mice, a failure to adjust gene expression in response to rising blood glucose levels led to increased metabolic stress and beta cell failure. The study was published in the journal Diabetes.
To investigate the mechanisms of beta cell loss in T2D, researchers at the DZD performed single-cell RNA sequencing of islets of Langerhans in two obese mouse strains that differ in their susceptibility to diabetes. Both the diabetes-prone and diabetes-resistant mice have six different groups of beta cells in their islets, which are very similar in abundance before treatment.
However, after feeding a carbohydrate-rich diabetogenic diet for two days, does levonorgestrel affect your period the composition of the beta cell clusters differed significantly between the strains. The islet cells of the diabetes-resistant mice developed into a protective beta cell cluster (Beta4). This protective cluster showed indications of reduced beta cell identity (such as downregulation of the GLUT2, GLP1R and MafA genes).
Characteristics of mature beta-cell expression decreased. This likely leads to lower glucose uptake and for some, even gain of the ability to divide to ultimately produce more beta cells. An in vitro knockdown of GLUT2 in beta cells led to reduced stress reactions and a decrease in apoptosis markers (apoptosis = programmed cell death). This could explain the improved survival of beta cells in diabetes-resistant mice.
In contrast, beta cells from diabetic-prone mice responded with expression changes indicative of metabolic pressure and stress in the endoplasmic reticulum. They also lacked the adaptation of gene expression towards a more dedifferentiated state. This can presumably contribute to a later loss of beta cells, which in turn contributes to the development of diabetes.
Source: Read Full Article