telmisartan from cipla
<img class="aligncenter" src="https://scx1.b-cdn.net/csz/news/800a/2023/blood-factor-can-turn.jpg"
alt="Blood factor can turn back time in the aging brain"
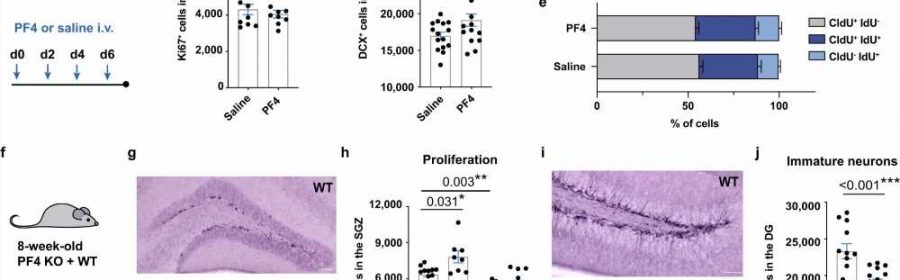
title="Systemic PF4 enhances adult hippocampal neurogenesis in vivo. a Experimental design of PF4 injection paradigm in young mice. b, c Intravenous (i.v.) PF4 injections for 1 week did not affect neural precursor cell proliferation in the subgranular zone (SGZ; n = 10 mice in saline group; n = 9 mice in PF4 group; counts of one hemisphere), but increased the number of doublecortin+ (DCX+) cells (n = 15 mice per group; counts of one hemisphere). d Experimental design of the double-labeling paradigm with CldU and IdU. e Acute administration of PF4 did not affect neural precursor cell proliferation, including the recruitment of cells from quiescence (n = 20 mice per group). f Running paradigm of PF4 knockout (KO) mice. g Representative images of Ki67+ cells in the SGZ of PF4 KO and wildtype (WT) mice. Scale bar: 50 μm. h PF4 KO mice show a significant reduction in the number of proliferating cells compared to wildtype mice. Physical exercise did not increase neural precursor proliferation in PF4 KO mice (WT STD n = 12; WT RUN n = 8; KO STD n = 14; KO RUN n = 8; counts of one hemisphere). i Representative images of DCX+ cells in the dentate gyrus (DG) of PF4 KO and wildtype mice. Scale bar: 50 μm. j PF4 KO mice have significantly lower levels of baseline neurogenesis compared to wildtype littermates (WT STD n = 12; KO STD n = 14; counts of one hemisphere). STD standard-housing, RUN 10-day running. Bars are mean ± SEM. Statistical analysis was performed using unpaired Student’s two-tailed t tests in (c) and (j), labetalol i.v and one-way ANOVA with Sidak comparison in (h). *p < 0.05, **p < 0.01, ***p < 0.001. Source data are provided as Source Data file. Credit: Nature Communications (2023). DOI: 10.1038/s41467-023-39873-9″ width=”800″ height=”455″>
Platelets are behind the cognitive benefits of young blood, exercise and the longevity hormone klotho. In a remarkable convergence, scientists have discovered that the same blood factor is responsible for the cognitive enhancement that results from young blood transfusion, the longevity hormone klotho, and exercise.
In a trio of papers appearing in Nature, Nature Aging and Nature Communications, two UCSF teams and a team from the University of Queensland (Australia), identify platelet factor 4 (PF4) as a common messenger of each of these interventions.
As its name suggests, PF4 is made by platelets, a type of blood cell that alerts the immune system when there is a wound and helps to form clots. It turns out that PF4 is also a cognitive enhancer. Under its influence, old mice recover the sharpness of middle age and young mice get smarter.
“Young blood, klotho, and exercise can somehow tell your brain, ‘Hey, improve your function,'” said Saul Villeda, Ph.D., associate director of the UCSF Bakar Aging Research Institute and the senior author on the Nature paper. “With PF4, we’re starting to understand the vocabulary behind this rejuvenation.”
Villeda led the study on young blood, which was published in Nature. Dena Dubal, MD, Ph.D., UCSF professor and David A. Coulter Endowed Chair in Aging and Neurodegenerative Disease, led the study on klotho, which was published in Nature Aging. Tara Walker, Ph.D., professor of neuroscience at the University of Queensland, led the study on exercise, which was published in Nature Communications.
They committed to releasing their findings at the same time to make the case for PF4 from three different angles.
“When we realized we had independently and serendipitously found the same thing, our jaws dropped,” Dubal said. “The fact that three separate interventions converged on platelet factors truly highlights the validity and reproducibility of this biology. The time has come to pursue platelet factors in brain health and cognitive enhancement.”
Platelets quell the inflammation of an aging brain and body
Villeda is an expert on parabiosis, an experiment in which two animals are linked together by their blood circulation. When a young, sprightly animal is connected to an aging animal, the aging animal becomes more youthful–its muscles more resilient, its brain more capable of learning.
In 2014, Villeda found that plasma, consisting of blood minus red blood cells, mimicked parabiosis: young blood plasma, injected into old animals, was restorative. When his team compared young plasma to old plasma, they found it contained much more PF4.
Just injecting PF4 into old animals was about as restorative as young plasma. It calmed down the aged immune system in the body and the brain. Old animals treated with PF4 performed better on a variety of memory and learning tasks.
“PF4 actually causes the immune system to look younger, it’s decreasing all of these active pro-aging immune factors, leading to a brain with less inflammation, more plasticity and eventually more cognition,” Villeda said. “We’re taking 22-month-old mice, equivalent to a human in their 70s, and PF4 is bringing them back to function close to their late 30s, early 40s.”
Platelets ferry klotho’s signal for cognitive enhancement into the brain
A decade ago, Dubal, a member of the UCSF Weill Neurosciences Institute, showed that klotho enhances cognition in young and old animals and also makes the brain more resistant to age-related degeneration. But she knew its effects had to be indirect because klotho molecules, injected into the body, never reached the brain. Dubal’s team found that one connection was PF4, released by platelets after an injection of klotho.
PF4 had a dramatic effect on the hippocampus, the brain region responsible for making memories, where it enhanced the formation of new neural connections at the molecular level.
It also gave both old and young animals a brain boost in behavioral tests, suggesting that “there’s room to go even in young brains to improve cognitive function,” according to Dubal.
Other recent findings from Dubal have bolstered the prospects for using klotho therapeutically. Klotho’s benefits depend on the activation of platelets, leading to the release of PF4 and other molecules, which could each have their own benefits during aging.
“Ideally, we’ll have multiple shots on goal for one of our biggest biomedical problems, cognitive dysfunction, with the fewest side effects and the most benefit,” Dubal said.
Exercise also improves brain health via platelets
Exercise can keep the mind sharp for decades. Walker and her lab found that platelets released PF4 into the bloodstream following exercise. When she tested PF4 on its own, as Dubal and Villeda also had, it improved cognition in old animals.
“For a lot of people with health conditions, mobility issues or of advanced age, exercise isn’t possible, so pharmacological intervention is an important area of research,” Walker said. “We can now target platelets to promote neurogenesis, enhance cognition and counteract age-related cognitive decline.”
More information:
Adam B. Schroer et al, Platelet factors attenuate inflammation and rescue cognition in ageing, Nature (2023). DOI: 10.1038/s41586-023-06436-3. www.nature.com/articles/s41586-023-06436-3
Park, C. et al. Platelet factors are induced by longevity factor klotho and enhance cognition in young and aging mice, Nature Aging (2023). DOI: 10.1038/s43587-023-00468-0. www.nature.com/articles/s43587-023-00468-0
Odette Leiter et al, Platelet-derived exerkine CXCL4/platelet factor 4 rejuvenates hippocampal neurogenesis and restores cognitive function in aged mice, Nature Communications (2023). DOI: 10.1038/s41467-023-39873-9
Journal information:
Nature Communications
,
Nature
,
Nature Aging
Source: Read Full Article