singulair pediatric dose
Research led by the University of Tübingen, Germany, along with partners at Stanford University, Emory University and the Cincinnati Children’s Hospital Medical Center, U.S., has looked into infant immune responses following SARS-CoV-2 infections during the initial months of life.
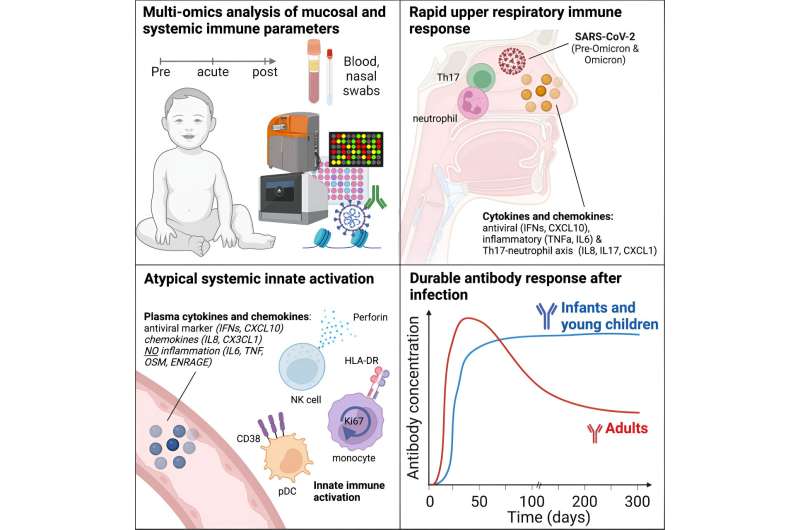
In a paper titled, “Multi-omics analysis of mucosal and systemic immunity to SARS-CoV-2 after birth,” published in Cell, the research team finds that infants and young children mount durable antibody responses for up to 300 days.
There has been a lack of comprehensive, nifedipine retard 30 mg system-wide, longitudinal analysis of how infants and young children respond to SARS-CoV-2 infection regarding their immune systems’ development, antibody responses, and innate immune activation. Researchers collected data on children, adults and mothers to get a comparative picture of infant immune reactions.
Blood and nasal swab samples were obtained from infants and young children in the IMPRINT cohort at the Cincinnati Children’s Hospital Medical Center. Children were tested weekly for SARS-CoV-2, and the cohort included 54 infected infants and young children, including 27 infants with paired pre-infection samples. An additional 27 matched control infants and young children represented healthy controls who consistently tested negative from birth to sampling.
In addition to the pediatric cohort, 62 blood samples were obtained from 48 adult COVID-19 patients and ten healthy control samples, collected from the Hope Clinic at Emory University in Atlanta and the Stanford University Medical Center. Blood samples were also obtained from 41 mothers with mild COVID-19, including three with paired pre-infection samples and three matched controls.
In contrast to adults, infants and young children displayed robust and durable antibody responses against SARS-CoV-2. These antibody titers remained high for up to 300 days, whereas antibody responses tend to decay more rapidly in adults.
In the blood, there was an upregulation of activation markers on innate cells in the children but no significant increase in inflammatory cytokines. Memory B and T cell responses in infants were significantly lower than in adults. However, they showed increases in multifunctional T helper 17 and 1-type CD4+ T cells characterized by the production of interleukin-2, interferon-gamma, and tumor necrosis factor-alpha, making them triple-positive.
Infants mounted a robust mucosal immune response characterized by inflammatory cytokines, interferon α, and markers associated with T helper 17 and neutrophil responses. This mucosal response was particularly pronounced in the nasal mucosa.
While multifunctional CD4 T cell responses were reduced by roughly two orders of magnitude in the infants, observations of persistent antibody responses lasted much longer. The study’s findings raise the possibility of designing vaccine formulations to take advantage of these innate immune system activation pathways to avoid causing the collateral immunopathology often associated with unwanted inflammation.
More information:
Florian Wimmers et al, Multi-omics analysis of mucosal and systemic immunity to SARS-CoV-2 after birth, Cell (2023). DOI: 10.1016/j.cell.2023.08.044
Journal information:
Cell
Source: Read Full Article