para q es nimotop
<img class="aligncenter" src="https://scx1.b-cdn.net/csz/news/800a/2023/atoh1-is-critical-to-e.jpg"
alt="Atoh1 is critical to establish the diversity of pontine nuclei neurons"
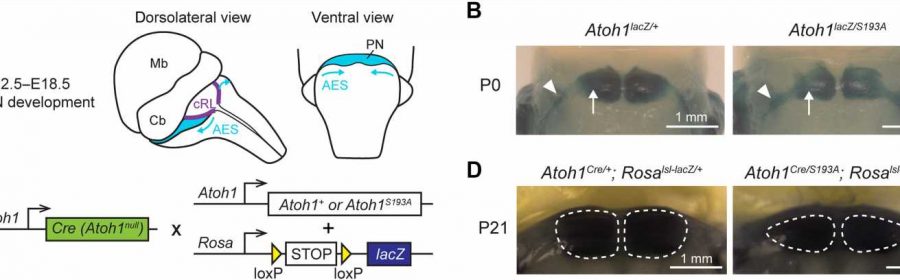
title="Phospho-mutation of Atoh1 at serine-193 leads to PN hypoplasia in mice. (A) Schematic representation of the PN development in mice. PN neurons migrate from cRL to ventral pons through AES during E12.5 to E18.5. Mb, midbrain; Cb, cerebellum. (B) Whole-mount X-galactosidase (X-gal) staining on mouse hindbrain at P0 (ventral view). The arrows and arrowheads denote the neurons at the PN and in the AES, respectively. Scale bars, 1 mm. (C) Strategy to label the Atoh1-lineage neurons. Atoh1Cre/+ knock-in mouse was crossed with mouse carrying either wild-type or hypomorphic Atoh1 (Atoh1S193A) and a Cre-dependent lacZ allele. (D) Whole-mount X-gal staining on mouse hindbrain at P21 (ventral view). The dashed lines outline the area of the PN. Scale bars, zofran safe in early pregnancy 1 mm. Credit: Science Advances (2023). DOI: 10.1126/sciadv.adg1671″ width=”800″ height=”362″>
A recent study published in Science Advances by researchers at Baylor College of Medicine and Texas Children’s Hospital has discovered six distinct neuronal lineages in the pons region of the brainstem and revealed new insights into their differential vulnerability to the partial loss of Atoh1, a gene crucial for the development of pontine neurons. The study was led by Howard Hughes Medical Institute investigator, Dr. Huda Y. Zoghbi, also a distinguished service professor at Baylor College of Medicine and founding director of the Jan and Dan Duncan Neurological Research Institute (Duncan NRI) at Texas Children’s Hospital.
“Each day we engage in a multitude of motor tasks which require intricate coordination between different brain regions,” said Sih-Rong Wu, a graduate student in the Zoghbi lab and first author of the study.
“Depending on the task, whether it is something as gentle as picking a flower or as hard as hitting a baseball with great force, our brain first needs to determine how much force is needed and then execute it correctly. Our ability to perform a diverse and complex range of motor movements is made possible by exquisite coordination between two brain regions—the cerebral cortex and cerebellum—which is orchestrated by pivotal groups of pontine nuclei neurons in the brainstem that relay and coordinate information rapidly and yet, with great precision.”
Atoh1 directs every step of pontine nuclei neuron formation, migration and maturation
In mammals, certain precursor “mother” cells in the developing hindbrain differentiate into pontine neurons primarily by the action of Atoh1. This gene, discovered by the Zoghbi lab, encodes a transcription factor, controls the expression of many genes, and regulates a multitude of critical biological functions ranging from breathing, balance, to hearing. However, its exact role in pontine neurons had been a mystery and it was unclear how a seemingly homogenous pool of Atoh1-expressing pontine nuclei progenitor cells direct the enormous variety of motor movements that humans and animals perform routinely.
To explore this question, the Zoghbi lab studied mice that lacked one copy of Atoh1 with another copy also being genetically modified, resulting in the partial loss of Atoh1 function. Interestingly, these mice not only showed a delay in the development of pontine neurons but also had a significantly smaller cluster of pontine neurons, which was reminiscent of pontine nuclei malformations seen in patients with Atoh1 mutations.
To investigate the role of Atoh1 in the development of pontine neurons, Wu and colleagues profiled the gene expression patterns of individual neurons in the developing hindbrain of control and mice with partial loss of Atoh1 function. They found Atoh1 gene function was critical for every stage of the formation of pontine neurons—from directing the production of mother cells, maintaining their survival, and promoting their migration, to differentiating into various neuronal subtypes.
To the team’s surprise, pontine nuclei “mother” cells in mice differentiated into six new and distinct subtypes in newborns. This was an unexpected finding because previous anatomical and connectivity studies of pontine nuclei had only identified two subtypes.
Furthermore, the team found that partial reduction of the Atoh1 function affects only a select population of pontine nuclei neurons, suggesting Atoh1 likely plays different roles in each of these subtypes, which might explain the differences in their vulnerabilities to its loss.
“This study provides a solid foundation to explore the enormous functional diversity of pontine nuclei neurons,” Dr. Huda Zoghbi said.
“The more we understand how specific types of neurons and circuits in different brain regions work and how they communicate with one another, the closer we get to decoding the complex ‘language’ of how the mammalian brain coordinates and directs complex motor and cognitive processes. Solving the fundamental puzzle of how different types of brain cells function individually and with each other is key to identifying safe and effective therapeutic approaches to correct brain circuit disturbances that arise in disease states.”
Others involved in the study were Jessica Butts, Matthew Caudill, Jean-Pierre Revelli, Ryan Dhindsa, and Mark Durham. They are affiliated with the Baylor College of Medicine and/or the Jan and Dan Duncan Neurological Research Institute at Texas Children’s Hospital.
More information:
Sih-Rong Wu et al, Atoh1 drives the heterogeneity of the pontine nuclei neurons and promotes their differentiation, Science Advances (2023). DOI: 10.1126/sciadv.adg1671
Journal information:
Science Advances
Source: Read Full Article