olmesartan o telmisartan
A promising new cancer therapy also appears extremely potent against one of the world’s most devastating infectious diseases: tuberculosis (TB).
Scientists at Texas Biomedical Research Institute (Texas Biomed) have found that the therapy dramatically reduces TB growth, even for bacteria that are drug-resistant. The findings, reported in the journal Biomedicine & Pharmacotherapy, were made in novel cellular models featuring TB-infected human cells that can help accelerate screening of potential TB drugs and therapies like this one.
The therapy evaluated in this study combines two molecules, one of which is already FDA-approved for use in cancer patients, and another that is being evaluated in Phase 1/2 clinical trials for cancer. The compounds help the body initiate its normal cell death processes in targeted areas, be it cancerous cells, or in this case, cells infected with Mycobacterium tuberculosis (M.tb), generic propranolol ca without prescription the bacteria that causes TB.
TB accounts for more than 1.6 million deaths worldwide every year. The bacterium primarily infects the lungs. Patients must take antibiotics for months to bring active infection under control; drug resistance is on the rise, making treatment even more challenging.
Dr. Schlesinger’s lab at Texas Biomed is focused on understanding the fundamental biological interactions between the airborne-transmissible bacteria and humans, and then using those insights to identify potential treatment targets.
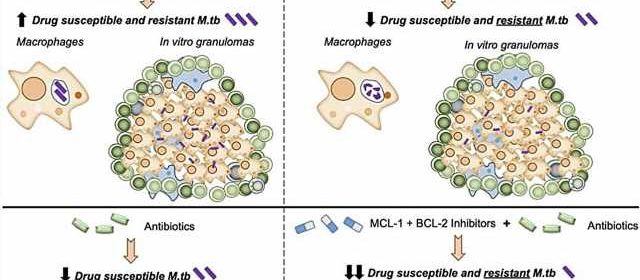
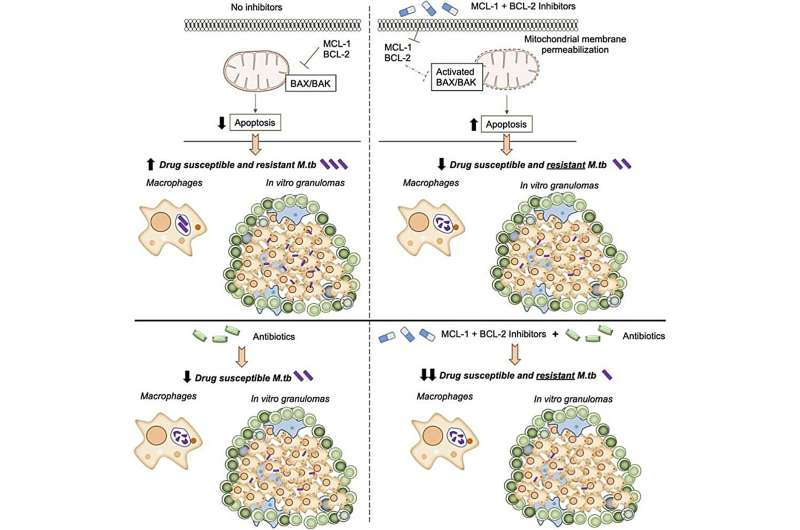
M.tb blocks a normal cell death process called apoptosis. This allows the bacteria to grow inside immune cells in the lungs, called alveolar macrophages. This new paper shows that by inhibiting two key proteins, MCL-1 and BCL-2, M.tb can no longer hijack the apoptosis process and macrophages are able to kill M.tb.
Importantly, this happens inside granuloma structures, the dense cellular clumps that the body forms around M.tb to try to contain it. Antibiotics and other treatments have a notoriously difficult time penetrating granulomas, which is one reason why M.tb is so hard to eliminate.
“Immunotherapy has been a gamechanger in the cancer field by finding ways to help a patient’s own immune system fight tumors more effectively,” says Larry Schlesinger, MD, Texas Biomed Professor, President and CEO, and senior paper author. “We believe that, similarly, host-directed therapies can be a gamechanger for infectious diseases.”
The research team, led by Texas Biomed Staff Scientist Eusondia Arnett, Ph.D., tested MCL-1 and BCL-2 inhibitors individually, together and in combination with TB antibiotics to see how TB growth was affected. Using both inhibitors was more effective at limiting TB growth than just one or the other; and combining them with antibiotics was far more effective than either inhibitors or antibiotics alone.
“The inhibitors combined with antibiotics controlled TB up to 98%, which is very exciting,” says Dr. Arnett, the first paper author. “But even more exciting is the inhibitors were just as effective at controlling drug-resistant TB as drug-susceptible TB. That is the power of a host-directed therapy targeting the human’s immune response versus trying to attack the pathogen directly.”
A key aspect of the research are the cellular models used to test the effectiveness of the inhibitors: human macrophages and a human granuloma model developed and refined in Dr. Schlesinger’s lab over the past decade. Human blood cells donated by volunteers are cultured with M.tb, which leads to the formation of granuloma-like structures.
“Granulomas are unique, dense environments that are not well replicated in mice,” Dr. Arnett says. “Our studies demonstrate that this cellular model can serve as an important bridge to identify compounds that can penetrate and retain activity in granulomas, before we move to the necessary—but more complex, time-consuming and expensive—animal research phase.”
Dr. Schlesinger and Dr. Arnett have filed a provisional patent for the combination therapy for infectious diseases. They are planning additional cell, mouse and nonhuman primate studies to gather further evidence about the therapy’s effectiveness and seek partnerships with industry collaborators. They are hopeful the therapy can move quickly to the clinic because years of safety studies have already been completed, or are underway, for the inhibitors for cancer applications.
More information:
Eusondia Arnett et al, Combination of MCL-1 and BCL-2 inhibitors is a promising approach for a host-directed therapy for tuberculosis, Biomedicine & Pharmacotherapy (2023). DOI: 10.1016/j.biopha.2023.115738
Journal information:
Biomedicine & Pharmacotherapy
Source: Read Full Article