levaquin and bactrim ds

Previous studies have described a wide range of clinical manifestations associated with post-acute sequelae of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (PASC) infection.
In a recent study published on the medRxiv* preprint server, researchers identify symptoms and systematic conditions associated with PASC in children. The researchers also investigated the medications used to treat children and adolescents with PASC one to six months after recovering from the coronavirus disease 2019 (COVID-19), along with identifying risk factors associated with PASC in this patient population.
 Study: Clinical features and burden of post-acute sequelae of SARS-CoV-2 infection in children and adolescents: an exploratory EHR-based cohort study from the RECOVER program. Image Credit: FamVeld / Shutterstock.com
Study: Clinical features and burden of post-acute sequelae of SARS-CoV-2 infection in children and adolescents: an exploratory EHR-based cohort study from the RECOVER program. Image Credit: FamVeld / Shutterstock.com
Background
PASC is defined as the persistence or relapse of symptoms, or the emergence of new health issues, after recovery from SARS-CoV-2 infection. These symptoms persist for a prolonged period after the initial infection. Researchers have observed that some of the clinical symptoms of PASC are more serious than others.
Many studies have characterized PASC in adults with respect to persistent and relapsing of non-specific symptoms such as headache, fatigue, and shortness of breath. However, there remains a lack of evidence describing PASC in children, define isosorbide apart from studies describing multisystem inflammatory syndrome in children (MIS-C).
Thus, there is an urgent need to understand the clinical manifestations and duration of PASC in children. This information could help standardize the definition and data collection methods related to PASC in children.
About the study
The current retrospective cohort study is part of the United States National Institutes of Health (NIH) Researching COVID to Enhance Recovery (RECOVER) Initiative, which primarily focuses on the treatment and preventive measures of PASC.
In this study, researchers obtained electronic health record (EHR) data from PEDSnet, which is a multi-institutional clinical research network that contains EHR data from many of the nation’s prime children’s healthcare facilities. PEDSnet contains relevant data of more than eight million in-patient and outpatient pediatric patients.
Study findings
Similar clinical manifestations were reported in children and adolescents with PASC as adults with this condition. Some of the symptoms included chest pain, changes in taste or smell, cardiorespiratory symptoms, fever, and fatigue. Interestingly, several other features were specifically observed in the pediatric population, some of which included hair loss, abnormal liver enzymes, diarrhea, and skin rashes.
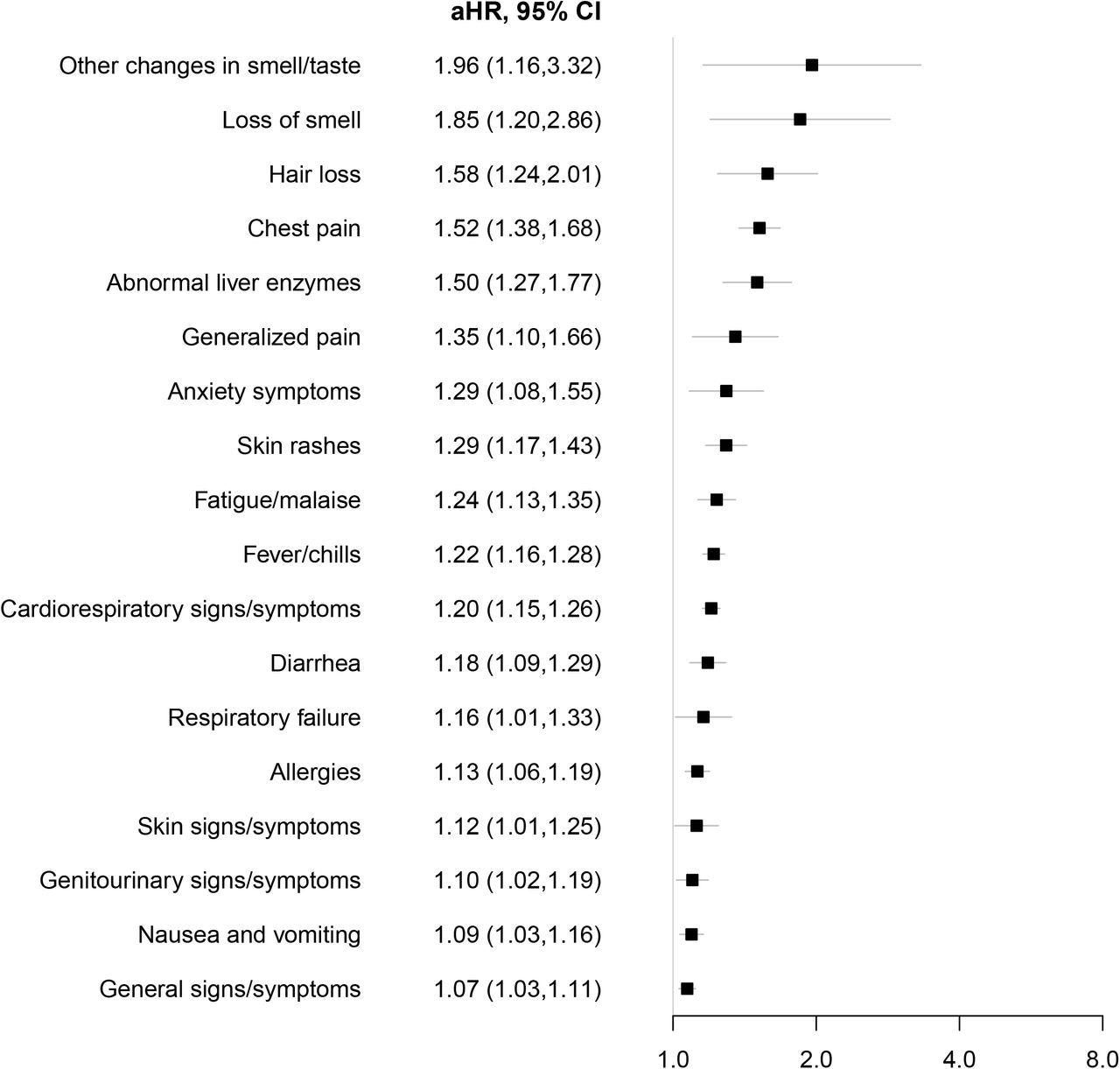 Syndromic PASC features associated with SARS-CoV-2 infection. Adjusted hazard ratios (aHR) with associated 95% CI among patients who tested positive for SARS-CoV-2 infection versus those who tested negative for the risk of each syndromic feature using Cox proportional hazards models. Models were adjusted for age at cohort entrance, sex, race/ethnicity, institution, testing place location, presence of a complex medical condition and date of cohort entrance.
Syndromic PASC features associated with SARS-CoV-2 infection. Adjusted hazard ratios (aHR) with associated 95% CI among patients who tested positive for SARS-CoV-2 infection versus those who tested negative for the risk of each syndromic feature using Cox proportional hazards models. Models were adjusted for age at cohort entrance, sex, race/ethnicity, institution, testing place location, presence of a complex medical condition and date of cohort entrance.
An increased rate of tonsillitis, pneumonia, and bronchiolitis was reported in children infected with SARS-CoV-2. In regard to systemic features, myocarditis also had a robust association with SARS-CoV-2 infection. This condition has been identified as an important complication in the pediatric population.
The researchers estimated that the burden of PASC that is not related to MIS-C is 3.7%. For this estimation, they computed the occurrence proportion difference of clinically predicted and empirically supported PASC features between the COVID-19-positive and negative groups.
This proportion reflects any systematic-, symptomatic-, or medication-related PASC features in the study cohort. However, this estimate must be regarded as a preliminary assessment, as the calculation was solely based on EHR data, which depends on clinicians’ coding practices.
The likelihood of PASC in SARS-CoV-2-infected children is associated with disease severity. This finding is consistent with previous studies, which indicate that the risk of PASC increased with acute COVID-19.
A lower incidence of PASC was reported in children as compared to adults. This finding differs from previous studies, which have reported a greater frequency of PASC in children. Notably, these earlier studies were prone to systematic selection biases of cases due to the absence of a relevant control group.
The measurable burden of PASC differs between adults and children because of the lack of evidence for recognizing symptoms associated with PASC in children, as well as the scarcity of documents that elucidate the difference in the immune response in children associated with PASC.
Strengths and limitations
One of the key strengths of this study is that the cohort included children and adolescents who were confirmed to be positive for COVID-19 based on a reverse transcription-polymerase chain reaction (RT-PCR) test. Similarly, the control group contained RT-PCR-negative children, which minimized bias.
Another strength is the inclusion of children from varied geographical locations across the U.S. Additionally, the study cohort included both outpatients, as well as hospitalized patients.
One of the limitations of this study is the EHR-based study cohort, which is dependent on clinicians’ practices. Therefore, the study cohort might have missed some relevant records that are stored in the laboratory, radiology department, and unstructured text data. Thus, the true burden of PASC might have been underestimated based on the EHR data.
Another limitation of this study is the inclusion of individuals in the control group who tested positive for COVID-19 outside the considered health system. Ethnicity-specific risk factors were also not determined for PASC, despite the fact that COVID-19 has disproportionally impacted minorities.
*Important notice
medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
- Rao, S., Lee, G. M., Razzaghi, H., et al. (2022). Clinical features and burden of post-acute sequelae of SARS-CoV-2 infection in children and adolescents: an exploratory EHR-based cohort study from the RECOVER program. medRxiv. doi:10.1101/2022.05.24.22275544. https://www.medrxiv.org/content/10.1101/2022.05.24.22275544v1.full-text
Posted in: Child Health News | Medical Science News | Medical Research News | Medical Condition News | Disease/Infection News | Healthcare News
Tags: Adolescents, Bronchiolitis, Chest Pain, Children, Coronavirus, Coronavirus Disease COVID-19, Diarrhea, Electronic Health Record, Fatigue, Fever, Frequency, Hair, Hair Loss, Headache, Healthcare, Immune Response, Laboratory, Liver, Myocarditis, Pain, Pneumonia, Polymerase, Polymerase Chain Reaction, Radiology, Research, Respiratory, SARS, SARS-CoV-2, Severe Acute Respiratory, Severe Acute Respiratory Syndrome, Skin, Syndrome, Tonsillitis, Transcription

Written by
Dr. Priyom Bose
Priyom holds a Ph.D. in Plant Biology and Biotechnology from the University of Madras, India. She is an active researcher and an experienced science writer. Priyom has also co-authored several original research articles that have been published in reputed peer-reviewed journals. She is also an avid reader and an amateur photographer.
Source: Read Full Article