ismo test papers 2013
<img class="aligncenter" src="https://scx1.b-cdn.net/csz/news/800a/2023/novel-cause-of-brain-m.jpg"
alt="Novel cause of brain mosaicism and focal epilepsy identified"
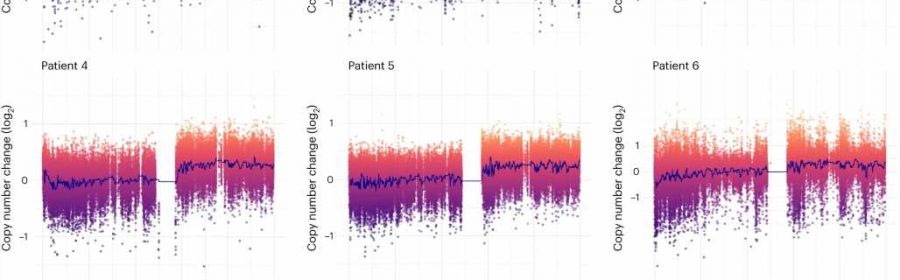
title="Brain mosaic copy number gain of chr1q. a, Six patients included in the study met the following criteria: infantile spasms progressing to intractable focal epilepsy, cortical dysplasia and hyaline astrocytic inclusions. Tissue obtained from surgical resection was evaluated for mosaicism using whole exome sequencing, FISH and single-cell WGS. b, Copy number analysis of exome sequencing data comparing brain tissue to a matched blood sample from each patient. Each patient had a non-integer (that is, mosaic) gain encompassing almost the entire long arm of chromosome 1 (1q21–q44). c, Representative examples of FISH analysis of patient brain cells showing three or four copies of chr1q present in each cell. Cells with 1q gain were observed on at least three slides per patient. d, cheap zithromax online canada now Copy number profiling of single nuclei from patient 3 brain tissue confirming the presence of chr1q tetrasomy in approximately 30% of nuclei. Credit: Nature Genetics (2023). DOI: 10.1038/s41588-023-01547-z” width=”800″ height=”530″>
In most people, every cell in their body contains the same genetic information. However, sometimes people can have two or more genetically different sets of cells. This usually happens during fetal development and is known as mosaicism. Sometimes one of those groups of cells has genetic changes that can cause diseases or disorders.
Neurologists, neurosurgeons and genomics experts have been working together to test for mosaicism in brain tissues resected during epilepsy surgery. Research has shown that mosaicism in the brain is a significant contributor to epilepsy.
In a new study, recently published in Nature Genetics, researchers from Nationwide Children’s Hospital describe an alternate origin of brain mosaicism in some children with focal epilepsy.
“About 1 in every 26 people will have epilepsy at some point in their lives. The causes are incredibly diverse, and a mystery in over half of people with epilepsy,” says Adam Ostendorf, MD, a pediatric neurologist at Nationwide Children’s and an author of the study.
About a third of children with epilepsy have drug-resistant seizures that greatly affect their quality of life, safety and developmental outcomes. “We were motivated to study the genetic causes of drug-resistant epilepsy so future research might be able to develop more effective treatments,” says Tracy Bedrosian, Ph.D., senior author of the paper and principal investigator in the Steve and Cindy Rasmussen Institute for Genomic Medicine at Nationwide Children’s Hospital.
The researchers performed a genetic analysis of brain tissue, blood and buccal (epithelial) cells. Tissue samples were from six patients, ages 2 months to 7 months, who underwent epilepsy surgery. The brain samples showed that some of the cells in the brain tissue (including astrocytes) had extra copies of chromosome 1q compared to the normal tissue. The blood and buccal cells did not have any cells with extra copies.
Notably, astrocytes carrying the extra 1q showed distinct gene expression signatures and histopathologic differences such as hyaline inclusions. This evidence supports the association of chromosome 1q gains with astrocytic inclusions in epilepsy.
“From a practical standpoint, in neurosurgical cases of focal epilepsy where we see certain histopathological signs, namely hyaline astrocytic inclusions, we can suspect the underlying genetic etiology is chromosome 1q gain,” says Dr. Bedrosian, who is also an assistant professor of Pediatrics at The Ohio State University College of Medicine.
But that etiology wasn’t the only finding in the study.
“The surprising finding was that the genetic alteration (chromosome 1q gains) was inherited in some of the patients, even though it was only found in a mosaic pattern within brain tissue,” Dr. Bedrosian adds. “This finding was a serendipitous observation that led us down a path to discover a novel mechanism for brain mosaicism.”
Analysis indicated that in five of the six patients, the extra chromosome copies were maternally derived—that is, they were present as a result of an error in the meiotic divisions that formed the egg. The analysis demonstrates that this error was corrected (rescued) by cell repair mechanisms during embryonic or fetal development in most tissues (blood and buccal) but not in all brain cells.
Katherine Miller, Ph.D., first author of the study and principal investigator in the Institute for Genomic Medicine, notes the uniqueness of the finding.
“We were able to genetically analyze blood from the mothers and then confirm that the extra chromosomal material was actually inherited, indicating a complex genetic phenomenon that resulted in the somatic mosaicism,” Dr. Miller says.
Normally, mosaicism is expected to be the result of changes to the genetic material during fetal development. However, these data demonstrate an alternate mechanism of brain chromosomal mosaicism—where the increase in copy number was inherited as the result of a meiotic error. During fetal development, these copy number gains were corrected in other cell lineages, making the copy number increase undetectable in blood and buccal cells.
“This work is incredibly exciting for two reasons,” says Dr. Ostendorf. “First, it links a recently identified cause to a pathology finding, furthering our understanding of how 1q gains cause unrelenting seizures. Second, it opens the door to new mechanisms of how brain tissue may be impacted by genetic problems differently than the rest of the body. Now, we have to reconsider how we look at genetic causes of epilepsy.”
“Recognizing a meiotic origin for mosaicism is also important for genetic counseling and risk of recurrence,” says Dr. Bedrosian. “If the chromosome gain is in additional tissues, patients may face an increased risk of cancers. Additionally, the mother’s future pregnancies might also be affected.”
More information:
Miller, K.E. et al, Post-zygotic rescue of meiotic errors causes brain mosaicism and focal epilepsy, Nature Genetics (2023). DOI: 10.1038/s41588-023-01547-z. www.nature.com/articles/s41588-023-01547-z
Journal information:
Nature Genetics
Source: Read Full Article