is xanax safe in early pregnancy

COVID-19, one of the deadliest pandemics in history, has been associated with more than 176 million infections and some 3.82 million confirmed deaths as of 16 June 2021.
Most countries have put in place phased vaccine distribution plans that prioritize those at the highest risk of complications, such as the elderly and those most exposed and at high risk for transmission, ventolin before running such as medical personnel.
Several COVID‑19 vaccines have demonstrated efficacy as high as 95% in preventing symptomatic COVID‑19 infections. Globally 17 vaccines are authorized by at least one national regulatory authority for public use. In total, as of March 2021, 308 vaccine candidates are in various stages of development.
Annual influenza vaccination is also a part of the public health recommendations in several countries as a preventative strategy to curb the seasonal epidemic that affects millions of people each year.
Globally, more than 2.4 billion doses of COVID-19 vaccine have been administered, and this ongoing mass vaccination campaign will undoubtedly coincide with seasonal flu vaccination campaigns.
The timing of booster COVID-19 vaccine doses in many countries will likely overlap with the 2021–2022 flu season in many settings. In addition, many other countries could still be administering the first doses of COVID-19 vaccines during the flu season.
There are currently insufficient data regarding the co-administration of COVID-19 vaccines with other vaccines. In order to formulate effective public health policies, it is essential to understand how co-administration affects safety and immune responses.
This is even more important in older adults as immunosenescence may leave them more vulnerable to seasonal influenza infection, related complications, and mortality and reduce their immune responses to regular influenza vaccines.
Evaluating the safety and efficacy of the NVX-CoV2373 vaccine when co-administered with the flu vaccine
Researchers from the UK recently reported the results of a sub-study that evaluates the safety, efficacy, and immunogenicity of the NVX-CoV2373 vaccine when co-administered with an approved seasonal influenza vaccine.
This sub-study was part of a phase 3 randomized UK trial of the safety and efficacy of the NVX-CoV2373 vaccine. Approximately 400 participants of the main trial who met the sub-study entry criteria and had no contraindications to the flu vaccine were chosen for the sub-study. This work is published on the medRxiv* preprint server prior to the peer review process.
The sub-study participants were randomized in a 1:1 ratio to receive NVX-CoV2373 (n=217) or placebo (n=214) and were given an approved, age-appropriate influenza vaccine along with the first dose of NVX-CoV2373.
Reactogenicity to the vaccines was determined with the help of an electronic diary for 7 days after vaccination. The participants were also monitored for medically attended AEs (MAAEs), unsolicited adverse events (AEs), and serious AEs (SAEs). In addition, SARS-CoV-2 anti-spike IgG and Influenza hemagglutination inhibition assays were performed. The authors also assessed vaccine efficacy against PCR-confirmed, symptomatic COVID-19 and made comparisons between sub-study and main study participants.
Flu vaccines are updated every year. We can learn from this process as we respond to COVID variants.
Findings show that concomitant vaccination did not alter the immune response to the seasonal influenza vaccine
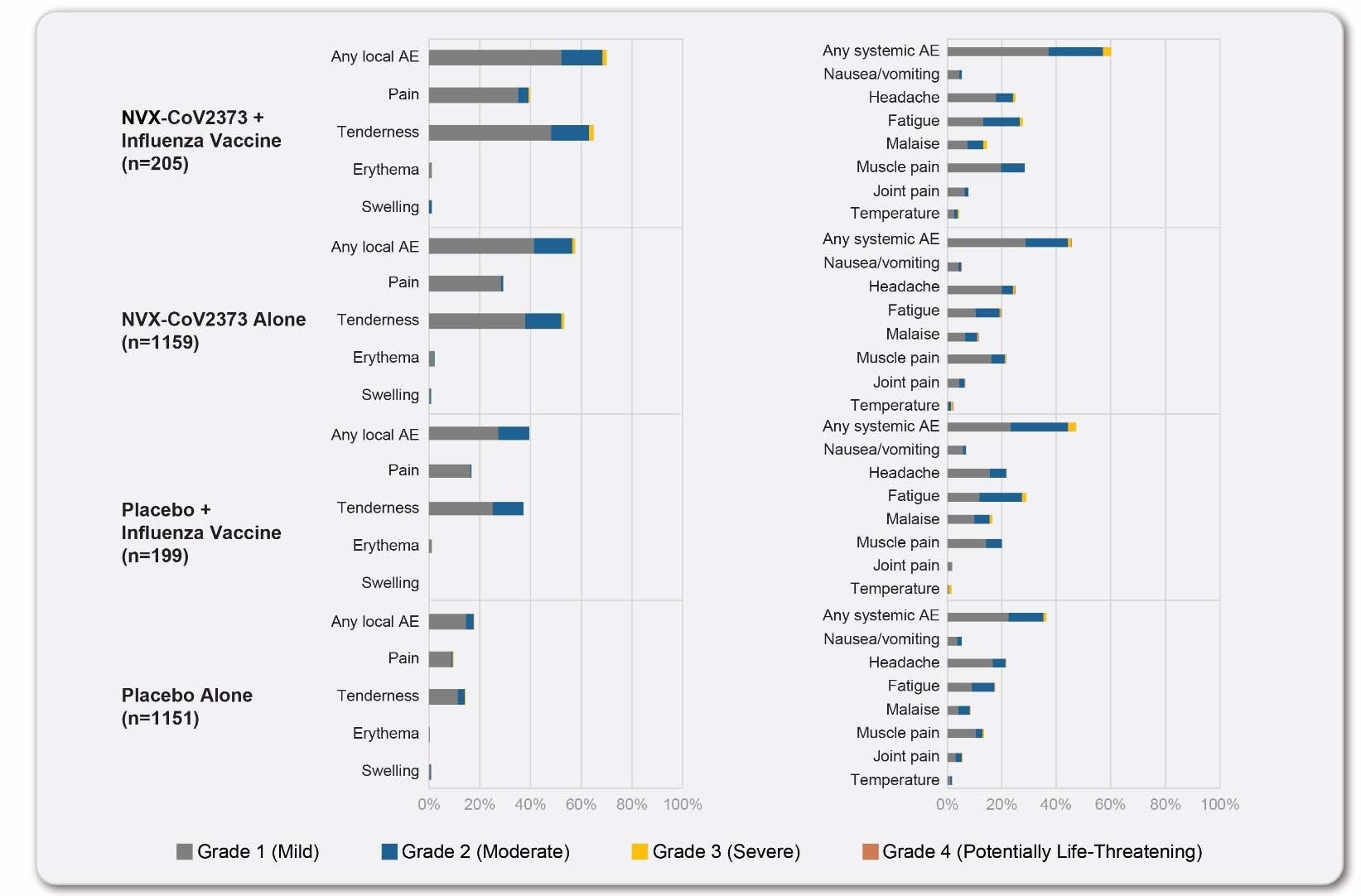
The participants of the sub-study were younger and more racially diverse compared to the main study participants. They also had fewer comorbid conditions. Reactogenicity events that were more common in the co-administration group were tenderness or pain at the injection site, fatigue, and muscle pain.
Unsolicited AEs, MAAEs, and SAEs were low and balanced among the two groups. While co-administration of the vaccines did not result in any change to the immune response to the seasonal influenza vaccine, a reduction was noted in the antibody response to the NVX-CoV2373 vaccine. While vaccine efficacy in the main study was 89.8%, efficacy in the sub-study was 87.5%.
Study suggests that co-administration of COVID and flu vaccines may be a viable immunization strategy
According to the authors, this is the first study demonstrating the safety, efficacy, and immunogenicity of a COVID-19 vaccine when co-administered with seasonal flu vaccines. The study's findings suggest that co-administration of COVID and flu vaccines is fairly safe and might be a viable immunization strategy.
Although no specific comparative immunogenicity endpoints were specified in this exploratory sub-study, the authors found no evidence for the COVID-19 vaccine interfering with the QIVc influenza vaccine. This study offers crucial information that can help guide the national immunization policy decision-making on concomitant use of COVID-19 vaccines with influenza vaccines. The authors hope that future clinical trials and studies of COVID-19 vaccines include safety and immunogenicity data on co-administration with other common pediatric and adult vaccines.
"More research on the concomitant vaccination of COVID-19 and influenza vaccines is needed, especially in those >65 years of age, to help guide national immunization policy on this critical issue."
*Important Notice
medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
- Safety, Immunogenicity, and Efficacy of a COVID-19 Vaccine (NVX-CoV2373) Co-administered With Seasonal Influenza Vaccines, Seth Toback, Eva Galiza, Catherine Cosgrove, James Galloway, Anna L. Goodman, Pauline A. Swift, Sankarasubramanian Rajaram, Alison Graves-Jones, Jonathan Edelman, Fiona Burns, Angela M. Minassian, Iksung Cho, Lakshmi Kumar, Joyce S. Plested, E. Joy Rivers, Andreana Robertson, Filip Dubovsky, Greg Glenn, Paul T. Heath, medRxiv, 2021.06.09.21258556; doi: https://doi.org/10.1101/2021.06.09.21258556, https://www.medrxiv.org/content/10.1101/2021.06.09.21258556v1
Posted in: Men's Health News | Medical Research News | Women's Health News | Disease/Infection News
Tags: Antibody, Coronavirus Disease COVID-19, Efficacy, Fatigue, Flu, Immune Response, Immunization, Influenza, Mortality, Muscle, Pain, Placebo, Public Health, Research, SARS, SARS-CoV-2, Vaccine

Written by
Susha Cheriyedath
Susha has a Bachelor of Science (B.Sc.) degree in Chemistry and Master of Science (M.Sc) degree in Biochemistry from the University of Calicut, India. She always had a keen interest in medical and health science. As part of her masters degree, she specialized in Biochemistry, with an emphasis on Microbiology, Physiology, Biotechnology, and Nutrition. In her spare time, she loves to cook up a storm in the kitchen with her super-messy baking experiments.
Source: Read Full Article