generic lipitor without prescription o
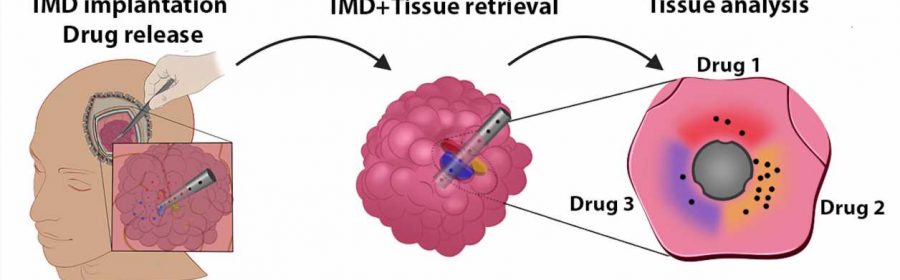
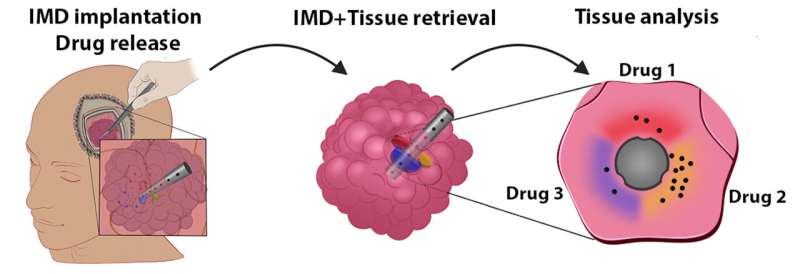
The shape and size of a grain of rice, the new device can conduct dozens of experiments at once to study the effects of new treatments on some of the hardest-to-treat brain cancers.
Researchers from Brigham and Women’s Hospital, a founding member of the Mass General Brigham health care system, have designed a device that can help test treatments in patients with gliomas, a type of tumor that originates in the brain or spinal cord.
The device, which is designed to be used during standard of care surgery, provides unprecedented insight into the effects of drugs on glioma tumors and caused no adverse effects on patients in a phase 1 clinical trial. Results from the pilot clinical trial for the device are published in Science Translational Medicine.
“In order to make the greatest impact on how we treat these tumors, oxycontin xanax combo we need to be able to understand, early on, which drug works best for any given patient,” said co-principal investigator and co-corresponding author Pierpaolo Peruzzi, MD, Ph.D., an assistant professor in the Department of Neurosurgery at Brigham and Women’s Hospital.
“The problem is that the tools that are currently available to answer this question are just not good enough. So we came up with the idea of making each patient their own lab, by using a device which can directly interrogate the living tumor and give us the information that we need.”
About 20,000 people in the U.S. each year are diagnosed with gliomas, a type of tumor that affects the brain and spinal cord. Gliomas are also among the deadliest brain cancers and are notoriously difficult to treat.
One challenge in developing targeted therapies for glioma is that it can be difficult to test many different combinations of drugs in tumor cells, because it’s only possible to treat patients with one approach at a time. This has been a significant barrier for hard-to-treat cancers like gliomas, for which combination therapies are a promising avenue.
Peruzzi worked closely with co-principal investigator Oliver Jonas, Ph.D., an associate professor in the department of Radiology at the Brigham, to develop a device that can work around some of the barriers to precision medicine in gliomas. These microdevices are implanted in a patient’s tumor during surgery and removed before the surgery is complete.
“It’s important that we are able to do this in a way that best captures the features of each patient’s tumor and, at the same time, is the least disruptive of the standard of care,” said Peruzzi. “This makes our approach easy to integrate into patients’ treatment and allows its use in real life.”
In the time the device is implanted—about two to three hours—it administers tiny doses of up to 20 drugs into extremely small areas of the patient’s brain tumor. The device is removed during the surgery and the surrounding tissue is returned to the lab for analysis.
Because the device works while the tumor is still in the body, conducting experiments this way gives unparalleled ability to assess the effects of drugs on the tumor microenvironment, the cells immediately surrounding cancer cells that can make up almost half the mass of a tumor.
“This is not in the lab, and not in a petri dish,” said Peruzzi. “It’s actually in real patients in real time, which gives us a whole new perspective on how these tumors respond to treatment.”
In the current study, the researchers tested their device on six patients undergoing brain surgery to remove a glioma tumor. None of the patients experienced any adverse effects from the device, and the researchers were able to collect valuable biological data from the devices, such as how the response changes based on drug concentrations, or what molecular changes each drug produces in the cells.
While the study demonstrated that the device was safe and could be easily incorporated into surgical practice, the researchers are still working on determining the exact ways the data it gathers should be used to optimize glioma therapy. The researchers are currently conducting a two-stage version of their procedure in which patients receive the device through minimally invasive surgery 72 hours before their main surgery.
“We’re optimistic that this is a new generation approach for personalized medicine,” said Peruzzi. “The ability to bring the lab right to the patient unlocks so much potential in terms of the type of information we can gather, which is new and exciting territory for a disease that has very few options at present.”
More information:
PierPaolo Peruzzi et al, Intratumoral drug-releasing microdevices allow in situ high throughput pharmaco phenotyping in patients with gliomas, Science Translational Medicine (2023). DOI: 10.1126/scitranslmed.adi0069. www.science.org/doi/10.1126/scitranslmed.adi0069
Journal information:
Science Translational Medicine
Source: Read Full Article