cleosin
A molecular signature that predicts aggressive thyroid cancer could help guide treatment approaches for patients, according to a study published in the journal Cell Genomics.
A multidisciplinary team from three medical centers studied samples from a large group of patients with thyroid cancer to better understand thyroid cancer progression and to identify biomarkers for aggressive disease.
The incidence of thyroid cancer is increasing, and by 2030 it is projected to be the fourth most common cancer diagnosed, klonopin vs l theanine said Vivian Weiss, MD, Ph.D., assistant professor of Pathology, Microbiology and Immunology at Vanderbilt University Medical Center and the corresponding author of the study. Surgery to remove part or all of the thyroid gland, sometimes followed by radioactive iodine treatment, is usually successful; the five-year survival rate is 98%.
Because thyroid cancer has such a high survival rate, it is a relatively understudied type of cancer, Weiss said.
“The problem is that some patients go on to have very aggressive disease, which is usually resistant to radioactive iodine,” Weiss said. “We don’t understand why some patients get progressive disease, and we have very limited treatment options for them.”
To explore how thyroid cancer becomes advanced—and returns in an aggressive form sometimes up to 10 years after initial treatment—Weiss and her colleagues assembled a large cohort of patients enriched for aggressive thyroid cancer.
They included samples from adult patients treated at VUMC and University of Washington over more than 15 years who had metastatic thyroid cancer or other aggressive types of thyroid cancer, including anaplastic thyroid cancer—a particularly lethal type that kills patients in months. They also included samples from patients who had malignant thyroid cancer without metastases or other forms of nonmalignant thyroid disease requiring surgery. The cohort, which is unique in its enrichment for aggressive thyroid cancer, included 251 patients with 312 samples.
The researchers, led by Vanderbilt graduate students George Xu and Matthew Loberg, sequenced DNA and RNA, analyzed spatial differences in gene expression, and used multiplex immunofluorescence to identify and visualize biomarkers of aggressive thyroid cancer.
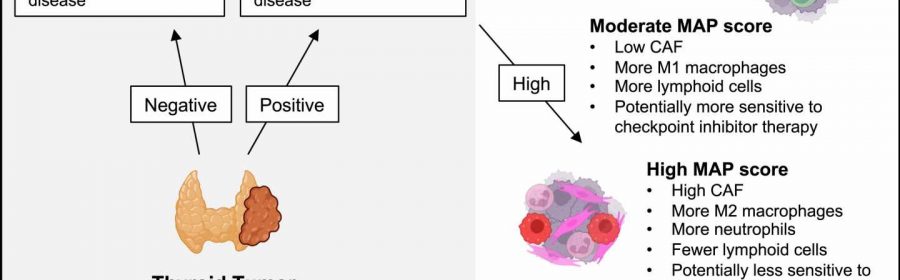
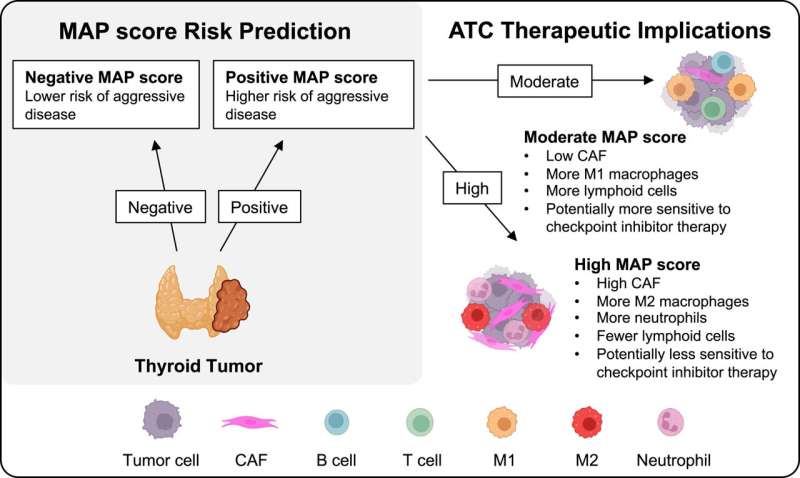
They identified a molecular signature—a collection of gene expression changes—associated with aggressive thyroid cancer and developed a Molecular Aggression and Prediction (MAP) score that can predict aggressive disease and poor clinical outcomes when combined with information about genetic mutations.
“An exciting feature of this cohort is that we have patients who have been treated for years, so we have follow-up samples and clinical information,” Weiss said. “With our MAP score, we were able to predict from a patient’s first surgery—before there was metastatic disease or any idea that the cancer would be aggressive—which patients would go on to have aggressive disease in the future.”
The MAP score also differentiated anaplastic thyroid cancer into types with different immune cell components—one that might respond to immunotherapy and another that likely will not respond.
“Being able to predict response to immunotherapy in patients with anaplastic thyroid cancer would be helpful to choose treatments as quickly as possible for this very lethal disease,” Weiss said.
Many of the genes in the molecular signature for aggressive thyroid cancer were associated with cells of the tumor microenvironment, such as immune cells and fibroblasts.
“Our work highlights differences in the tumor microenvironment across different thyroid cancers and provides us with ideas moving forward about how to better potentially treat this disease and understand its progression,” Weiss said. “Understanding the immune microenvironment in particular opens up potential strategies for therapy for patients when standard chemotherapy is not working and may help us identify new ways to target the immune system in these patients.”
In future studies, the researchers will continue to use spatial DNA/RNA sequencing across tumor tissue to explore how tumors progress from less to more aggressive.
Weiss emphasized the collaborative nature of the study and praised the talented students who carried out the work. Xu and Loberg are co-first authors of the Cell Genomics paper. They are joined by investigators from many departments at VUMC and Vanderbilt, and by researchers at the University of Washington School of Medicine and Johns Hopkins University School of Medicine.
More information:
George J. Xu et al, Molecular signature incorporating the immune microenvironment enhances thyroid cancer outcome prediction, Cell Genomics (2023). DOI: 10.1016/j.xgen.2023.100409
Journal information:
Cell Genomics
Source: Read Full Article