buy generic cialis professional no prescription mastercard

In a recent study published on the bioRxiv* preprint server, researchers demonstrate that the exposure of platelets to the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spike protein promotes their activation and adhesion, adderall side effects abuse thus enhancing calcium release and phosphatidylserine (PS) exposure to drive increased thrombin generation. The researchers also showed that the TMEM16F activity inhibitors of niclosamide and clofazimine almost completely eliminated this spike-induced pro-coagulant response.
The lung pathology of severe coronavirus disease 2019 (COVID-19) patients revealed thrombosis as a defining characteristic. Previous studies have shown that clinical indicators of thrombosis, including elevated D-dimer, fibrinogen, and thrombosis-associated inflammatory biomarkers, are present in most COVID-19 patients requiring intensive care.
Study: SARS-CoV-2 Spike protein activates TMEM16F-mediated platelet pro-coagulant activity. Image Credit: Kateryna Kon / Shutterstock.com
About the study
The present study was based on a few observations which suggested that thrombosis in COVID-19 is triggered by local events occurring in the infected lungs. The first observation is regarding the asynchronous deposition of thrombi and fibrin in the lungs. The deposition of these substances appears to be due to relatively recent thrombi infiltrated by inflammatory cells that are close to older thrombi in an advanced stage of fibrotic organization.
The second observation suggests that viral infection triggers local thrombotic events in the lungs due to the sporadic presence of macro- or microvascular thrombosis in other organs. The third observation opposes thrombosis as a result of a systemic consumptive coagulopathy since high D-dimer and fibrinogen levels have been identified in severe COVID-19 patients and no increase in prothrombin time or a decrease in antithrombin levels are observed. Various other observations emphasize the specific involvement of platelets in the pathogenesis of thrombosis in COVID-19 patients.
In the current study, the researchers produced SARS-CoV-2 spike or vascular stomatitis virus (VSV)-G protein-pseudotyped virions, or generated cells expressing the spike protein on their plasma membrane. Subsequently, the researchers examined their effects on platelet adhesion (fluorescence), aggregation (absorbance), exposure of phosphatidylserine (flow cytometry for annexin V binding), calcium flux (flow cytometry for fluo-4AM), and clot formation and retraction.
The researchers discovered a novel mechanism that regulates cell-cell fusion induced by the SARS-CoV-2 spike protein. They began with the observation that the lungs of almost 90% of COVID-19 patients contain several syncytia including two to more than 20 nuclei.
Screening two large libraries of European Medicines Agency (EMA)/ the United States Food and Drug Administration (FDA)-approved small molecules to search for drugs that inhibit spike-induced syncytia formation led to the identification of TMEM16F activity inhibitors niclosamide and clofazimine. Subsequent experiments on the aforementioned cell lines included an evaluation of how niclosamide and clofazimine treatment affects these cells.
“SARS-CoV-2 spike stimulated platelets both when present on the virion envelopes or upon expression onto the plasma membrane of cells.”
Study findings
The study data showed that the exposure of platelets to the SARS-CoV-2 spoke protein increases their activation and adhesion, as well as promotes the release of calcium from these cells. This increase in adhesion and aggregation occurred as a result of the spike protein’s effects on pro-coagulant platelet activation markers, including PS exposure on the platelet outer membrane and generation of thrombin.
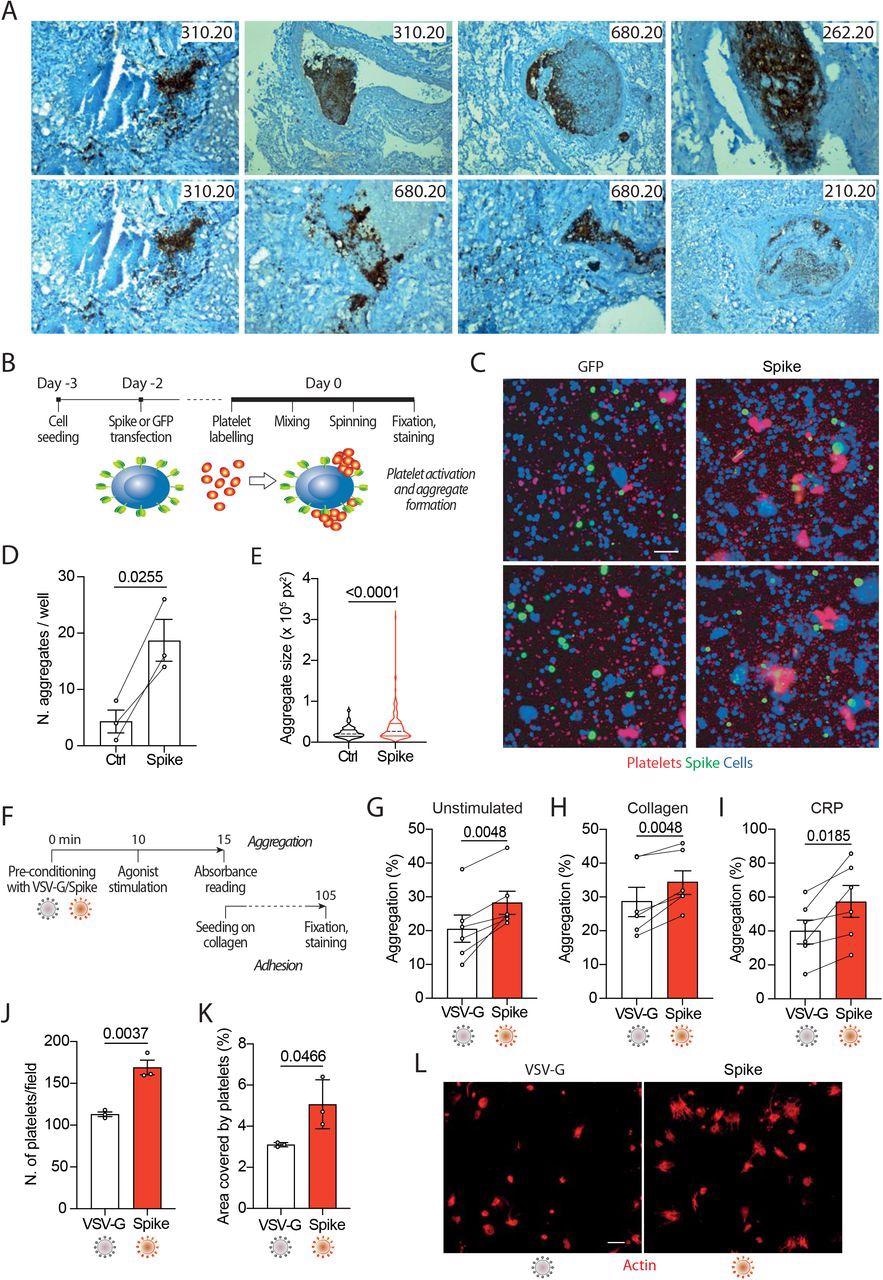
In severely infected SARS-CoV-2 patients, viral replication is robust in the lung and lower tract respiratory epithelium, which leads to the continuous production of infectious particles. Cells that were engineered to express the SARS-CoV-2 spike protein on their surface were found to fuse with neighboring cells expressing the angiotensin-converting enzyme 2 (ACE2) receptor, which is an essential component of the SARS-CoV-2 entry into host cells. This phenomenon subsequently leads to the formation of large syncytia, which have been observed in over 90% of patients with severe COVID-19.
Taken together, these findings suggest that cells infected with SARS-CoV-2, as well as those that contribute to the formation of large syncytia, contribute to platelet activation and the subsequent induction of thrombosis. Two possible mechanisms that could be responsible for this spike-mediated platelet activation were proposed.
The first potential mechanism suggests that platelet activation could occur directly upon binding of the spike protein to the ACE2 receptor that is expressed in platelets, thereby leading to direct activation of TMEM16F on the platelet plasma membrane. Comparatively, the activation of TMEM16F could be triggered by the increase in calcium that occurs following stimulation with the SARS-CoV-2 spike protein.
In agreement with the first proposed mechanism, the researchers found that the platelets were not activated when the medium was depleted of extracellular calcium, thus indicating that intracellular calcium stores are not required for activation. Platelet activation was also observed occurred upon treatment with isolated spike receptor-binding domain (RBD), which suggests that TMEM16F directly acts on the platelets following the binding of the SARS-CoV-2 spike protein to the ACE2 receptor.
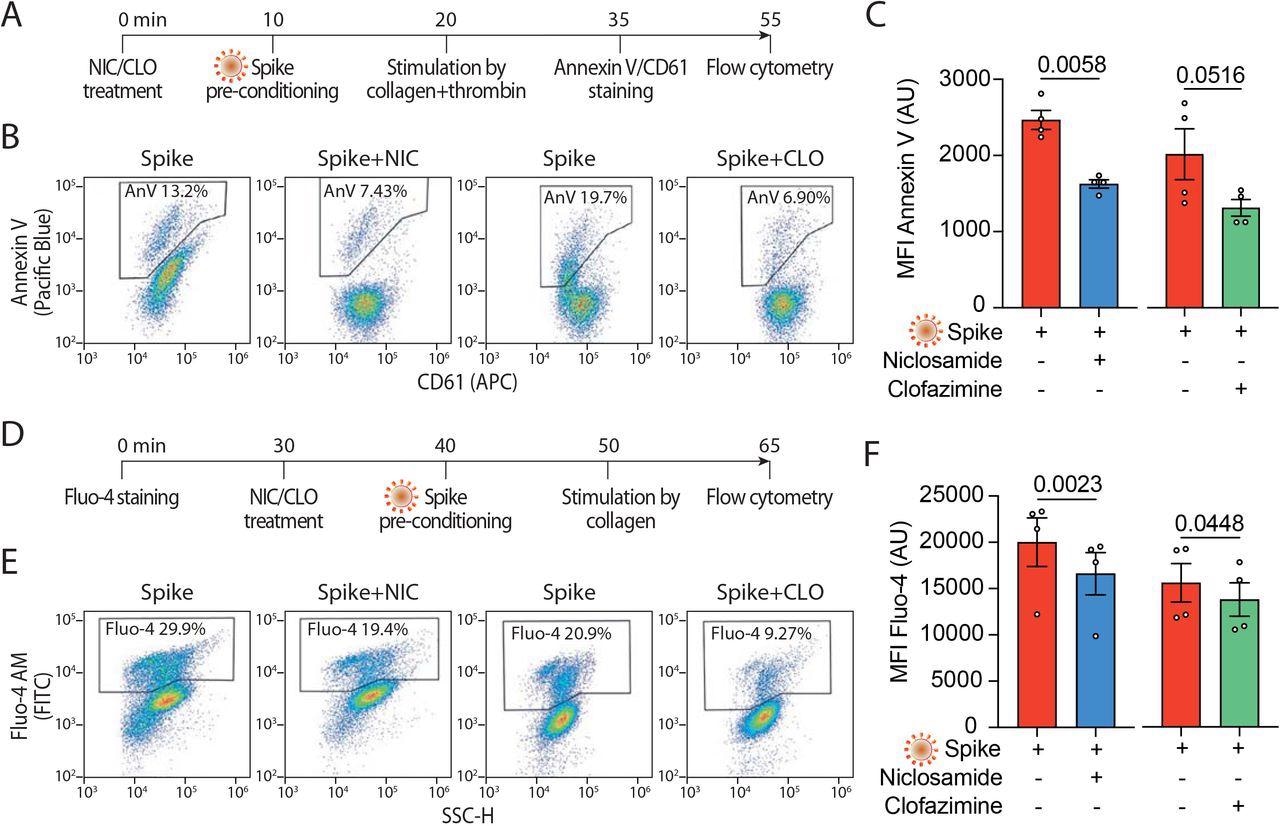
Both niclosamide and clofazimine act by blocking spike-induced syncytia formation in a variety of epithelial and non-epithelial cells expressing the ACE2 receptor. The experiments conducted in the current study using these agents found that both niclosamide and clofazimine effectively inhibited spike-induced platelet activation; however, niclosamide was particularly effective at inhibiting platelet activation at low concentrations.
The results of the current study provide evidence for a pathogenic mechanism that might be responsible for COVID-19-induced thrombosis. These findings also support the repurposing of niclosamide, which targets TMEM16F, for the treatment of COVID-19.
*Important notice
bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
- Cappelletto, A., Allan, H. E., Crescente, M., et al. (2021). SARS-CoV-2 Spike protein activates TMEM16F-mediated platelet pro-coagulant activity. bioRxiv. doi:10.1101/2021.12.14.472668 https://www.biorxiv.org/content/10.1101/2021.12.14.472668v2
Posted in: Medical Research News | Medical Condition News | Disease/Infection News
Tags: ACE2, Angiotensin, Angiotensin-Converting Enzyme 2, Calcium, Cell, Coronavirus, Coronavirus Disease COVID-19, Cytometry, D-dimer, Drugs, Enzyme, Flow Cytometry, Fluorescence, Food, Intensive Care, Intracellular, Lungs, Membrane, Pathology, Platelet, Platelets, Protein, Receptor, Respiratory, SARS, SARS-CoV-2, Severe Acute Respiratory, Severe Acute Respiratory Syndrome, Spike Protein, Stomatitis, Syndrome, Thrombosis, Vascular, Virus

Written by
Saurabh Chaturvedi
Saurabh Chaturvedi is a freelance writer from Jaipur, India. He is a gold medalist in Masters in Pharmaceutical Chemistry and has extensive experience in medical writing. He is passionate about reading and enjoys watching sci-fi movies.
Source: Read Full Article