anusol hc ointment 0.5

In most settings, currently available coronavirus disease 2019 (COVID-19) vaccines offer protection against hospitalization and death, as demonstrated by clinical trial data. However, estimates of real-world efficacy are affected by various factors including characteristics of the current circulating variant, population demographics, vaccine protocols, and time post-vaccination. There is currently an increased risk of breakthrough infections, partly due to immune waning.
 Study: Impact of SARS-CoV-2 infection on longitudinal vaccine immune responses. Image Credit: Christoph Burgstedt
Study: Impact of SARS-CoV-2 infection on longitudinal vaccine immune responses. Image Credit: Christoph Burgstedt
The generation of virus-specific antibodies and T-cell responses through the induction of memory B-cells and T-cells following infection or vaccination is the basis of a robust immune response. There has been an inverse correlation associated with antibody levels and risk of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. With the number of infections increasing globally, it will become more common for individuals to receive vaccines post-infection.
In a recent study published on the preprint server medRxiv*, a team of researchers utilized longitudinally collected blood samples from the COMMUNITY study. Through the use of these samples, alprazolam jenis narkoba the researchers reported the binding and pseudo-neutralizing antibody titers elicited over a period of seven months after the patients had received either the Pfizer-BioNTech mRNA BNT162b2 vaccine or three months following adenovirus-vectored ChAdOx1 nCoV-19 (AstraZeneca) vaccination in 517 healthcare workers with and without previous confirmed SARS-CoV-2 infection.
Study findings
Following vaccination, 99.8% of the participants exhibited detectable levels of spike immunoglobulin G (IgG) antibodies. When the recovered individuals were compared to the naïve, at all time points of the experiment, the recovered displayed higher spike IgG geometric mean titers (GMT).
In naïve participants, between week 6 and 12, there was a 2-fold decrease in spike IgG GMTs and between week 6 and week 29, a 6.6-fold decrease was observed. A decrease of 1.5-fold between week 6 and 12 and 3.6-fold between week 6 and 29 was also seen in the previously infected.
Against both the Delta and wildtype (WT) strains of SARS-CoV-2, binding spike IgG titers correlated strongly to pseudo-neutralizing antibody titers. When compared to naïve individuals, previously infected individuals displayed substantially higher GMTs of pseudo-neutralizing antibodies.
The GMTs among naïve participants against the Delta and WT strains between weeks 6 and 12 decreased by 1.8-fold and 2.6-fold, respectively. Furthermore, between weeks 6 and 29, the decreases were 3.3-fold and 4.6-fold, respectively.
In the participants who had experienced a SARS-CoV-2 infection prior to vaccination, there was a decrease in pseudo-neutralizing antibodies against the Delta and WT strains of 2.3-fold and 2.1-fold between weeks 6 and 12, respectively, and of 5.1-fold and 3.6-fold between weeks 6 and 29, respectively.
Notably, similar levels of pseudo-neutralizing GMTs for both the WT and Delta strains were observed. This observation suggests a similar trend in response to the currently circulating Delta variant as was for the WT.
When compared with the BNT162b2 vaccine, naïve individuals who received the ChAdOx1 vaccine exhibited levels of spike IgG GMTs that were 4.5-fold lower. Similar to what was seen with the BNT162b2 vaccine, previously infected individuals who received the ChAdOx1 vaccine showed an increase in spike IgG GMTs compared to naïve individuals.
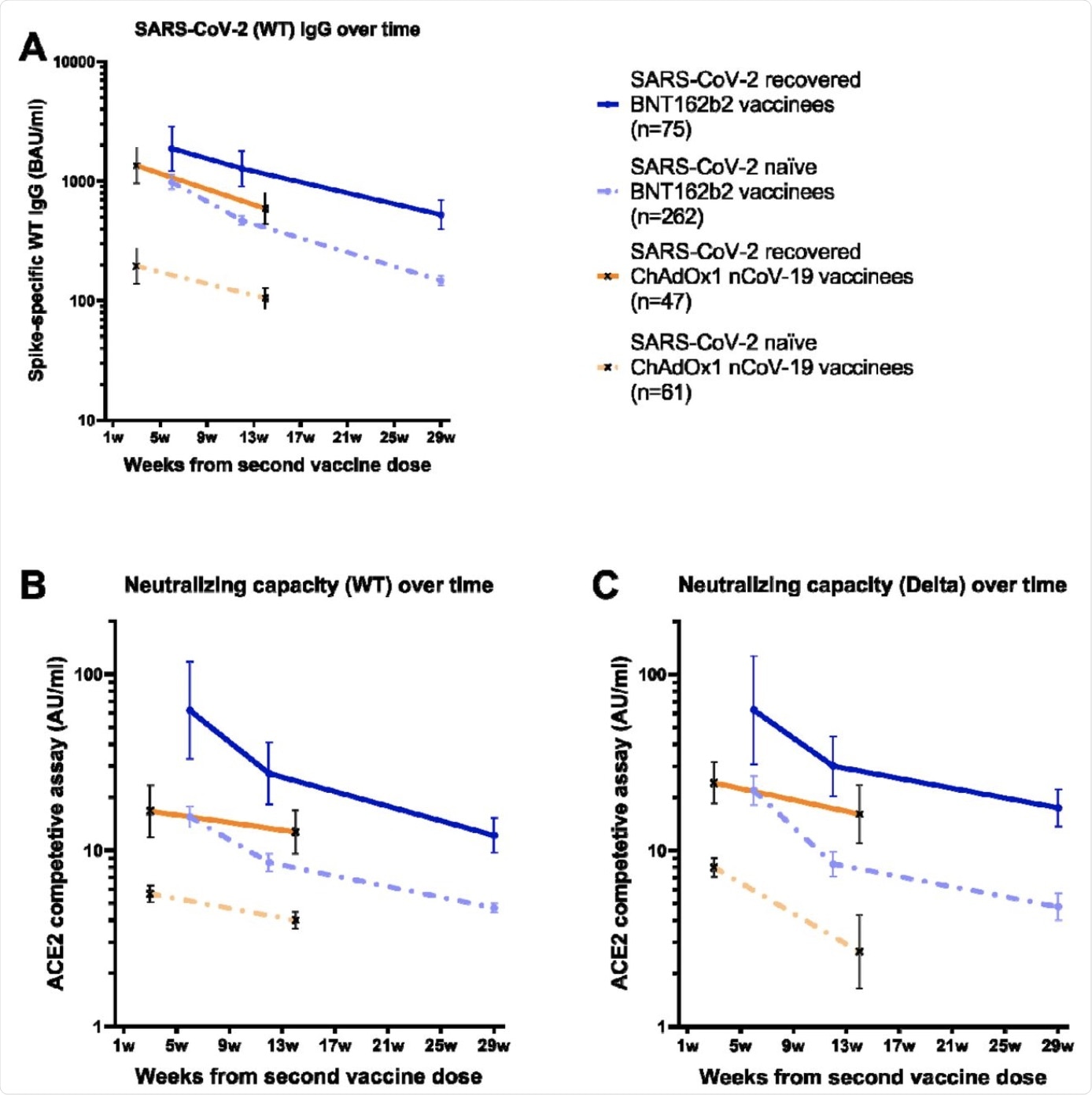 Binding and pseudo-neutralizing antibody titers over time following BNT162b2 and ChAdOx1 nCoV-19 vaccination with and without prior SARS-CoV-2 infection. A) Binding antibody titers against SARS-CoV-2 wild type over 7 months following the second BNT162b2 dose and 3 months following the second ChAdOx1 nCoV-19 dose in SARS-CoV-2 recovered and naïve vaccinees, B) pseudo-neutralizing antibodies against the wild type over 7 months following the second BNT162b2 dose and 3 months following the second ChAdOx1 nCoV-19 dose in SARS-CoV-2 recovered and naïve vaccinees, and C) pseudo-neutralizing antibodies against the Delta variant type over 7 months following the second BNT162b2 dose and 3 months following the second ChAdOx1 nCoV-19 dose in SARS-CoV-2 recovered and naïve vaccinees. Dots and crosses represent geometric mean titers and bars represent 95 % CI. Solid lines represent SARS-CoV-2 recovered vaccinees and dotted lines represent SARS-CoV-2 naïve vaccinees. WT; wild type, BAU; binding antibody units, AU; arbitrary units.
Binding and pseudo-neutralizing antibody titers over time following BNT162b2 and ChAdOx1 nCoV-19 vaccination with and without prior SARS-CoV-2 infection. A) Binding antibody titers against SARS-CoV-2 wild type over 7 months following the second BNT162b2 dose and 3 months following the second ChAdOx1 nCoV-19 dose in SARS-CoV-2 recovered and naïve vaccinees, B) pseudo-neutralizing antibodies against the wild type over 7 months following the second BNT162b2 dose and 3 months following the second ChAdOx1 nCoV-19 dose in SARS-CoV-2 recovered and naïve vaccinees, and C) pseudo-neutralizing antibodies against the Delta variant type over 7 months following the second BNT162b2 dose and 3 months following the second ChAdOx1 nCoV-19 dose in SARS-CoV-2 recovered and naïve vaccinees. Dots and crosses represent geometric mean titers and bars represent 95 % CI. Solid lines represent SARS-CoV-2 recovered vaccinees and dotted lines represent SARS-CoV-2 naïve vaccinees. WT; wild type, BAU; binding antibody units, AU; arbitrary units.
With the previously infected participants, spike IgG GMTs declined by 2.3-fold between weeks 3 and 14, whereas the GMTs of naïve individuals declined 1.9-fold between weeks 3 and 14. Consistent with the binding antibodies, individuals with a previous infection who received the ChAdOx1 vaccine showed substantially higher GMTs of pseudo-neutralizing antibodies when compared to infected naïve individuals.
In the naïve group who received the vaccine, by weeks 3 and 14, GMTs against the Delta and WT strains decreased by 1.4-fold and 3-fold, respectively. Comparatively, in the previously infected group, GMTs decreased by 1.3-fold and 1.5-fold, respectively. Similar to what was observed with the BNT162b2 vaccination, GMTs against the Delta variant reached corresponding levels as GMTs against the WT.
Implications
A decline in antibodies over a 3–7-month period is observed in the current study. This finding is coupled with the reports of waning vaccine effectiveness and correlates with the latest suggestion of a third booster vaccination, especially for those at a higher risk of severe complications associated with COVID-19. The strong impact that the previous infection has on vaccine effectiveness was also demonstrated in this study.
Participants with prior infections exhibited increased and more sustained levels of neutralizing antibodies when compared to the infection naïve. From these findings, it could be suggested that previous SARS-CoV-2 infections should be taken into consideration when planning booster doses and design of current and future vaccination programs.
*Important notice
medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
- Havervall, S., Marking, U., Greilert-Norin, N., et al. (2021). Impact of SARS-CoV-2 infection on longitudinal vaccine immune responses. medRxiv. doi:10.1101/2021.10.16.21264948. https://www.medrxiv.org/content/10.1101/2021.10.16.21264948v1
Posted in: Medical Research News | Disease/Infection News | Pharmaceutical News
Tags: Adenovirus, Antibodies, Antibody, Blood, Cell, Clinical Trial, Coronavirus, Coronavirus Disease COVID-19, Efficacy, Healthcare, Immune Response, Immunoglobulin, Respiratory, SARS, SARS-CoV-2, Severe Acute Respiratory, Severe Acute Respiratory Syndrome, Syndrome, T-Cell, Vaccine, Virus
.jpg)
Written by
Colin Lightfoot
Colin graduated from the University of Chester with a B.Sc. in Biomedical Science in 2020. Since completing his undergraduate degree, he worked for NHS England as an Associate Practitioner, responsible for testing inpatients for COVID-19 on admission.
Source: Read Full Article