ambien and other sleep medicines

A rare set of immune diseases called clonal mast cell activation disorders (cMCADs) may be associated with strong immunity to the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which is the pathogen responsible for the coronavirus disease 2019 (COVID-19).
An intriguing new study published on the preprint server bioRxiv* reports a year-long study in patients with cMCADs from all over France. In their study, no patient who was diagnosed with COVID-19 required non-invasive or mechanical ventilation, even when high-risk factors were present.
 Study: Absence Of Severe COVID-19 In Patients with Clonal Mast Cells Activation Disorders: Effective Anti-SARS-Cov-2 Immune Response. A Prospective And Comprehensive Study In France During One Year. Image Credit: Anuchit kamsongmueang / Shutterstock.com
Study: Absence Of Severe COVID-19 In Patients with Clonal Mast Cells Activation Disorders: Effective Anti-SARS-Cov-2 Immune Response. A Prospective And Comprehensive Study In France During One Year. Image Credit: Anuchit kamsongmueang / Shutterstock.com
Background
The cMCADs are a range of heterogeneous disorders characterized by the activation of one or multiple clones of mast cells, which is a well-known type of inflammatory cell. These disorders are characterized by the production of interferons (IFNs), including type I IFN, which is produced by plasmacytoid dendritic cells, as well as type III IFN (IFN-γ) from adaptive T-cells. Both these responses are prominent in antiviral immunity against SARS-CoV-2.
Mast cells trigger T-helper type 2 cells (Th2), alternative to yasmin which counteract the Th1 response that is responsible for identifying and destroying viruses and virus-infected cells. Moreover, mast cells release pro-inflammatory cytokines like interleukin (IL-)1, IL-6, and tumor necrosis factor (TNF). The breakdown of mast cell granules in the lungs may also worsen lung lesions.
For all these reasons, the current study was aimed at assessing the potentially increased risk of severe COVID-19 in patients with cMCADs.
About the study
The researchers exploited the French rare disease network CEREMAST to identify patients with cMCAD who also had COVID-19, as confirmed by a positive polymerase chain reaction (PCR) test or serology. Sera from these patients were analyzed for T-cell reactivity by measuring IFN-γ from peripheral blood mononuclear cells (PBMCs) after stimulating them with different pools of SARS-CoV-2 peptides.
Of the 32 patients included in the current study, approximately 60% were female, with the median age being about 50 years. About 40% of participants were at high risk for severe COVID-19 due to factors such as a high body mass index (BMI), immunocompromised condition, or cardiovascular disease. Notably, two participants were diabetic.
Most of the participants in the current study were being treated symptomatically with histamine receptor antagonists, or other mast cell antagonists. Most study participants became seropositive during the follow-up period.
Study findings
Fever was the most common COVID-19 symptom, with over half reporting anosmia/ageusia. Only two of the patients had to be hospitalized, w none were on invasive or non-invasive ventilation.
Interestingly, 25% of patients exhibited signs of mast cell activation during COVID-19, whereas 9.4% reported a reduction in this parameter. Overall, no severe COVID-19 was observed, even in the high-risk subset.
This study is remarkable for its comprehensive inclusion of every patient with advanced cMCAD who contracted severe symptomatic SARS-CoV-2 infection in France. Almost all patients in the T-cell study arm showed a specific T-cell response to one or more of the viral peptide pools, but at low intensities.
The responses were similar to control patients without these mast cell conditions, but with mild-to-moderate COVID-19, with the exception of the spike N-terminal peptide fragment pool. This showed lower reactivity in the cMCAD patients.
When compared to severe COVID-19 patients, this pattern of lower T-cell-specific reactivity was seen in the cMCAD patients. Such a difference was not seen between the groups when tested for T-cell-specific responses to the endemic coronavirus spike proteins, thus indicating that there was no defect in the response to these coronaviruses with mast cell disorders.
Specific immunoglobulin G (IgG) and IgA antibodies were also tested, showing that only one of 15 tested patients had a negative IgG test. In most cases, neutralizing IgG responses were detected; therefore, this group had a high prevalence of antibodies to SARS-CoV-2 with high-titer neutralizing antibodies.
An interesting finding was the occurrence of spontaneous IFN-γ release without stimulation of mast cells, though the exact cell of origin could not be identified. This could be due to higher baseline fractions of activated CD4+ T-cells and CD8+ T-cells, or due to the role of natural killer (NK) cells in keeping this baseline level of activation high.
One hypothesis puts NK cells at the center of allergic inflammation due to the unbalanced activation of cytokines in atopic individuals. In the current study, IFN-γ-secreting activated NK1 cells were reduced.
In an intriguing first, serum tryptase levels were also linked to the spontaneous IFN-γ release in some patients with cMCADs. This observation may partly explain why clonal mast cell numbers are associated with IFN-γ release in patients in the early stages of some of these conditions. This may also confer more protection against severe viral diseases.
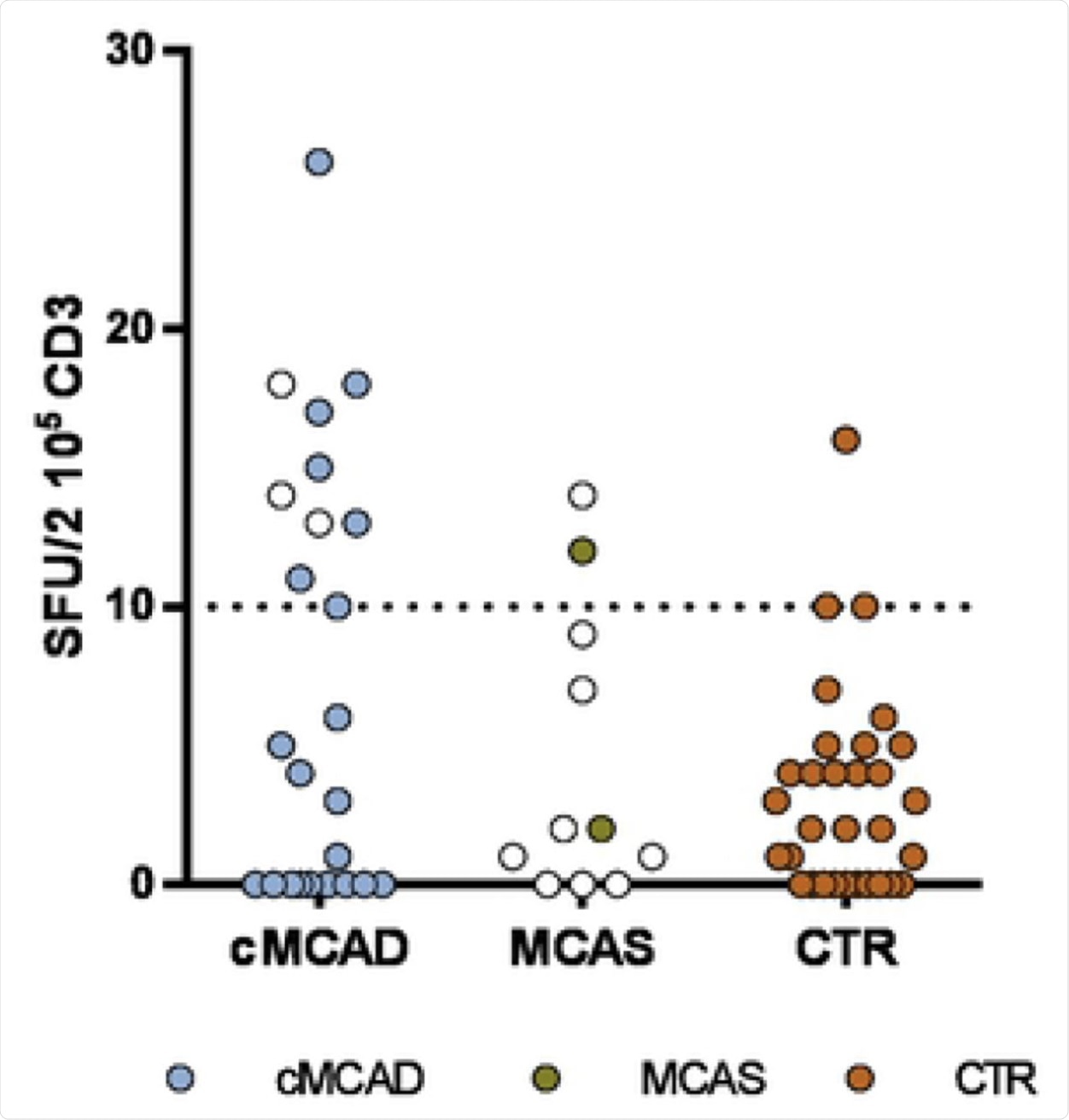 A. cMCADs: patients with clonal Mast Cells Activation Disorders. MCAS: patients with idiopathic mast cell activation syndrome. CTR: convalescent controls without cMCADs or MCAS. Empty circle: no COVID-19. Filled circle: history of COVID-19. Pictures of EliSpot assays. B: Well with non-stimulated PBMC from COVID-19 control without cMCADs. C: Well with non-stimulated PBMC from COVID-19 cMCADs patient. D: Well with PBMC from COVID-19 control without cMCADs after stimulation for 18-20h using individual 15-mers 11-aa overlapping peptide pools derived from SARS-CoV-2 N-terminal fragment Spike protein. E: Well with PBMC from COVID-19 cMCADs patient after stimulation for 18-20h using individual 15-mers 11-aa overlapping peptide pools derived from SARS-CoV-2 N-terminal fragment Spike protein.
A. cMCADs: patients with clonal Mast Cells Activation Disorders. MCAS: patients with idiopathic mast cell activation syndrome. CTR: convalescent controls without cMCADs or MCAS. Empty circle: no COVID-19. Filled circle: history of COVID-19. Pictures of EliSpot assays. B: Well with non-stimulated PBMC from COVID-19 control without cMCADs. C: Well with non-stimulated PBMC from COVID-19 cMCADs patient. D: Well with PBMC from COVID-19 control without cMCADs after stimulation for 18-20h using individual 15-mers 11-aa overlapping peptide pools derived from SARS-CoV-2 N-terminal fragment Spike protein. E: Well with PBMC from COVID-19 cMCADs patient after stimulation for 18-20h using individual 15-mers 11-aa overlapping peptide pools derived from SARS-CoV-2 N-terminal fragment Spike protein.
Implications
The researchers believe that patients with cMCADs can mount a protective Th1-cell immune response against SARS-CoV-2. This is reassuringly contrary to the expectations of many scientists who thought that the involvement of mast cells in Th2-skewed immune responses put these patients at a high risk of severe COVID-19 because of the potentially impaired Th1 antiviral response.
The T-cell-specific response to SARS-CoV-2 showed no difference in reactivity between patients with cMCADs and controls, though the magnitude of the response against the viral spike protein was somewhat lower in the former group. The activated mast cells may have reduced the Th1 response, a finding which may impact the efficacy of COVID-19 vaccines in this group.
Conversely, it is quite possible that mast cells may favor the balance between Th1/Th2 that prevents COVID-19 patients from progressing to severe disease.
The link between serum tryptase and IFN-γ release was reported here for the first time and appears to be protective against severe viral inflammation. This requires further study, as it could be exploited to treat patients with severe infectious conditions.
The presence of cMCADs was consistent with effective protection against SARS-CoV-2-related severe disease. However, all patients became seronegative upon follow-up at a median of 33 weeks. This indicates that COVID-19 vaccines are recommended in this group, but with the proviso that their effectiveness must be confirmed.
“Non-advanced mastocytosis and monoclonal mast cells activation syndrome most likely do not confer an increased risk for severe COVID-19.”
If the link between spontaneous IFN-γ release and protection against severe COVID-19 is confirmed, it may unravel one mechanism of immunity against this virus.
*Important notice
bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
- Rossignol, J., Ouedrani, A., Livideanu, C. B., et al. (2021). Absence Of Severe COVID-19 In Patients with Clonal Mast Cells Activation Disorders: Effective Anti-SARS-Cov-2 Immune Response. A Prospective And Comprehensive Study In France During One Year. bioRxiv. doi:10.1101/2021.09.01.458516. https://www.biorxiv.org/content/10.1101/2021.09.01.458516v1
Posted in: Medical Research News | Medical Condition News | Disease/Infection News
Tags: Anosmia, Antibodies, Blood, Body Mass Index, Cardiovascular Disease, CD4, Cell, Coronavirus, Coronavirus Disease COVID-19, Cytokines, Efficacy, Fever, Histamine, Immune Response, immunity, Immunoglobulin, Inflammation, Interferons, Interleukin, Lungs, Mast Cell, Mastocytosis, Necrosis, Pathogen, Peptides, Polymerase, Polymerase Chain Reaction, Protein, Rare Disease, Receptor, Respiratory, SARS, SARS-CoV-2, Serology, Severe Acute Respiratory, Severe Acute Respiratory Syndrome, Spike Protein, Syndrome, T-Cell, Tumor, Tumor Necrosis Factor, Virus

Written by
Dr. Liji Thomas
Dr. Liji Thomas is an OB-GYN, who graduated from the Government Medical College, University of Calicut, Kerala, in 2001. Liji practiced as a full-time consultant in obstetrics/gynecology in a private hospital for a few years following her graduation. She has counseled hundreds of patients facing issues from pregnancy-related problems and infertility, and has been in charge of over 2,000 deliveries, striving always to achieve a normal delivery rather than operative.
Source: Read Full Article