SARS-CoV-2 RBD is a selective integrin agonist

In a recent study published on the bioRxiv* preprint server, researchers assess the Arg-Gly-Asp (RGD) motif-mediated binding of the receptor-binding domain (RBD) of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spike (S) protein subunit (S1) and cell surface integrins (integrin receptors). The current study also investigates whether the integrin-S1 RBD binding could activate pathways of downstream signaling.
 Study: Receptor-binding domain of SARS-CoV-2 is a functional αv-integrin agonist. Image Credit: Design_Cells / Shutterstock.com
Study: Receptor-binding domain of SARS-CoV-2 is a functional αv-integrin agonist. Image Credit: Design_Cells / Shutterstock.com
Background
Integrins have been essential in the pathophysiology of several pulmonary viruses such as cytomegalovirus, influenza virus, and hantaviruses as primary receptors or key mediators of host responses and infection severity. However, the contributions of integrins in COVID-19 have not been extensively investigated.
The identification of a conserved integrin-binding RGD motif within the SARS-CoV-2 S protein has generated significant interest. To this end, several studies have reported that integrin inhibition may protect against SARS-CoV-2 binding to the host angiotensin-converting enzyme 2 (hACE2) receptor, thereby preventing coronavirus disease 2019 (COVID-19).
About the study
In the present work, researchers investigated the functional interactions of the SARS-CoV-2 S1 with αv integrins using primary human small airway epithelial cells (hSAECs) and fibronectin-null mouse embryonic fibroblasts (FN-null MEFs). They also compared the effects of integrin blocking antibodies on the cellular attachment to RBD.
To evaluate the functional interaction between S1-RBD and cells, FN-null MEFs were inserted into wells that were coated with RGD-containing fibronectin module, FNIII10, or S1-RBD. The team assessed the ability of S1-RBD to facilitate cellular proliferation, for which FN-null MEFs were inserted onto tissue culture plates that were coated with FNIII10, S1-RBD, or glutathione S-transferase (GST).
After four days of incubation, cell numbers were quantified to indicate the coating concentrations. Additionally, FN-null MEFs were also incubated with blocking antibodies against αv, β1, α5, β3 integrin subunits to identify the integrins that mediate S1-RBD cell adhesion. Further, competitive inhibition assays were carried out with RAD-, KGD-, or RGD-containing peptides.
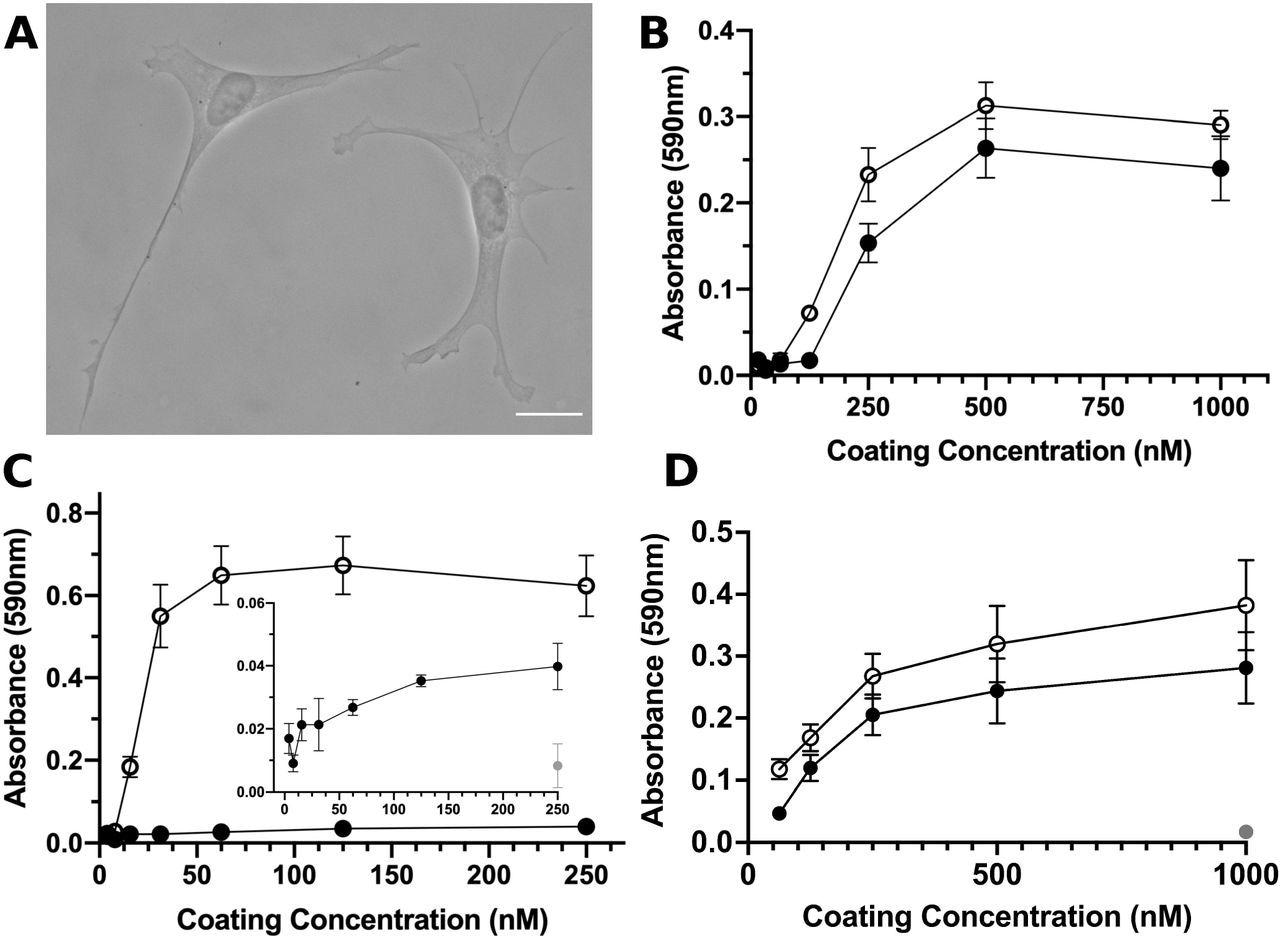 S1-RBD supports cell adhesion and proliferation. A, FN-null MEFs (2.5×10 cells/cm) were seeded onto coverslips pre-coated with S1-RBD (500 nM) and cultured for 4 h prior to fixation and phase-contrast imaging. Scale bar, 20 μm. B, FN-null MEFs (1.9×105cells/cm2) were seeded onto tissue culture plates precoated with the indicated concentration of S1-RBD (filled circles) or FNIII10 (open circles). Cells were cultured for 90 min and relative cell number was determined by crystal violet staining. C, FN-null MEFs (1.9×105 cells/cm2) were seeded onto plates pre-coated with HN-tagged S1 (filled circles) or FNIII8-13 (open circles) for 90 min. Inset shows cell adhesion to S1 (filled circles) compared to BSA-coated wells (gray circle). D, FN-null MEFs (2.3×103 cells/cm2) were seeded onto tissue culture plates pre-coated with the indicated concentration of S1-RBD (filled circles), FNIII10 (open circles), or GST (gray circle) and cultured for 4 d. Relative cell number was determined by crystal violet staining. Data are mean ± SEM; n ≥ 3 experiments performed in triplicate.
S1-RBD supports cell adhesion and proliferation. A, FN-null MEFs (2.5×10 cells/cm) were seeded onto coverslips pre-coated with S1-RBD (500 nM) and cultured for 4 h prior to fixation and phase-contrast imaging. Scale bar, 20 μm. B, FN-null MEFs (1.9×105cells/cm2) were seeded onto tissue culture plates precoated with the indicated concentration of S1-RBD (filled circles) or FNIII10 (open circles). Cells were cultured for 90 min and relative cell number was determined by crystal violet staining. C, FN-null MEFs (1.9×105 cells/cm2) were seeded onto plates pre-coated with HN-tagged S1 (filled circles) or FNIII8-13 (open circles) for 90 min. Inset shows cell adhesion to S1 (filled circles) compared to BSA-coated wells (gray circle). D, FN-null MEFs (2.3×103 cells/cm2) were seeded onto tissue culture plates pre-coated with the indicated concentration of S1-RBD (filled circles), FNIII10 (open circles), or GST (gray circle) and cultured for 4 d. Relative cell number was determined by crystal violet staining. Data are mean ± SEM; n ≥ 3 experiments performed in triplicate.
Surface plasmon resonance (SPR) was subsequently used for kinetic modeling of the S1-RBD and integrin-binding. FN-null MEF cells that adhere to the S1-RBD were stained with phalloidin, which is an actin-binding protein, and antibodies against vinculin, which is a focal adhesion molecule, and a phosphotyrosine antibody to determine whether engagement of αvβ3 integrins by S1-RBD triggers downstream signaling and supports focal adhesion formation.
The cells were immunoblotted to identify proteins that specifically phosphorylate by the S1-RBD ligation. These immunoblots were subsequently probed with phosphospecific antibodies against key components of adhesion signaling pathways. The recombinant S1 protein was chemically reduced by dithiothreitol (DTT) to determine whether the RGD motif was cryptic or not.
Study findings
Post-four-hour-seeding, S1-RBD supported adhesion of both FN-null MEFs and hSAECs, which showed classical fibroblast morphology. The cellular adhesion to S1-RBD was RGD- and cation-dependent and was inhibited by the blocking antibodies against αvβ3-integrin-specific ligands and FNIII10, but not β1 or α5 integrins. Using SPR, direct binding of S1-RBD with αvβ6 and αvβ3 integrins, but not α5β1 integrins, was noted.
The addition of RGD peptides inhibited cell adhesion to S1-RBD and FNIII10, whereas KGD-peptides did not reduce S1-RBD adhesion. The cell numbers increased with rising coating concentrations on the FNIII10- and S1-RBD-coated wells. Comparatively, cells seeded into GST-coated wells did not survive. This indicates the specificity of the cell proliferation in response to S1-RBD.
Kinetic modeling of the binding of αvβ6 integrin with S1-RBD provided a KD value of 230 nM, thus indicating that the S1-RBD directly binds to αv integrins with low-affinity interactions. The binding affinities of αvβ6 and αvβ3 with FNIII10 were 6.6 nM and 21.8 nM, respectively.
The rates of association for αvβ6 interactions with FNIII10 and S1-RBD were similar of 7.1 x 104 M-1s-1 and 8.4 x 104 M-1s-1, respectively. Contrastingly, the rates of dissociation of αvβ6 and S1-RBD were substantially greater than those with FNIII10 with the kd of 6.0 x10-4 s -1 for FNIII10 and 236 x 10-4 s-1 for S1-RBD.
In the tyrosine phosphorylation analysis, FAK-Y397, Src-Y418, Akt, and paxillin were phosphorylated on S1-RBD ligation to a similar extent in FNIII10- and hSAECs. This indicates that S1-RBD ligation supports cell proliferation and can trigger adhesion-mediated intracellular signaling pathways and activate downstream adhesive effectors such as paxillin and Akt.
Robust cell adhesion and spreading were observed for S1-RBD and FNIII10, with no differences in the absence or presence of MnCl2. The chemical reduction of S1 and pre-treatment of cells with Mn2+ partially rescued cell adhesion to S1. This indicates that the RGD motif of S1-RBD was cryptic, could be exposed by activating integrins, reduced by disulfide, and would be available to integrins only under particular chemical and physical conditions.
Taken together, the study findings demonstrate that the RGD motif within S1-RBD is an αvβ3 and αvβ6-selective integrin agonist. This supports the possibility that S1-RBD-mediated dysregulation of extracellular matrix (ECM) kinetics may play a role in the pathogenesis and/or post-acute sequelae of COVID-19.
*Important notice
bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
- Norris, E. G., Pan, X. S., & Hooking, D. C. (2022). Receptor-binding domain of SARS-CoV-2 is a functional αv-integrin agonist. bioRxiv. doi: 10.1101/2022.04.11.487882. https://www.biorxiv.org/content/10.1101/2022.04.11.487882v1.
Posted in: Molecular & Structural Biology | Medical Science News | Medical Research News | Disease/Infection News
Tags: Actin, Angiotensin, Angiotensin-Converting Enzyme 2, Antibodies, Antibody, Cation, Cell, Cell Adhesion, Cell Proliferation, Coronavirus, Coronavirus Disease COVID-19, covid-19, Cytomegalovirus, Enzyme, Fibroblast, Imaging, Influenza, Intracellular, Ligation, Molecule, Morphology, Pathophysiology, Peptides, Phosphorylation, Proliferation, Protein, Receptor, Respiratory, SARS, SARS-CoV-2, Severe Acute Respiratory, Severe Acute Respiratory Syndrome, Syndrome, Tissue Culture, Tyrosine, Virus

Written by
Pooja Toshniwal Paharia
Dr. based clinical-radiological diagnosis and management of oral lesions and conditions and associated maxillofacial disorders.
Source: Read Full Article