Researchers create new type of COVID-19 antibody test

As the COVID-19 pandemic continues with many thousands of new infections reported each day, there is a need for widely applicable surveillance testing to gain a better understanding of infection rates, especially the number of infections in people with mild or no symptoms, who can still be carriers. UNC School of Medicine scientists and colleagues developed a new kind of antibody test—a simplified experimental assay that could be ramped up to test thousands of blood samples at labs that do not have the resources of commercial labs and large academic medical centers.
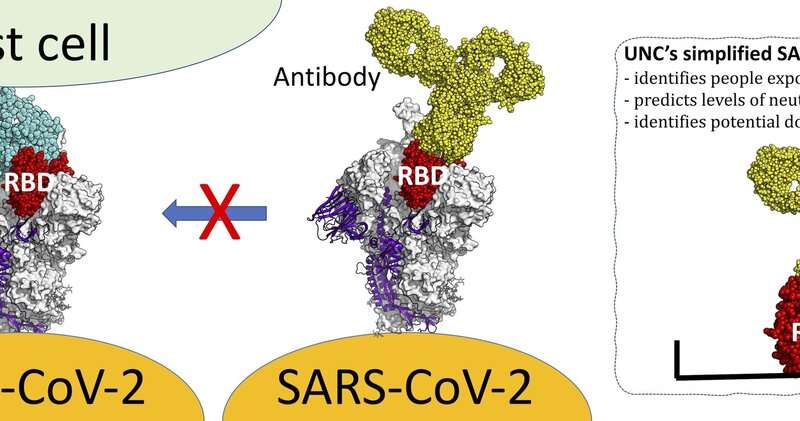
The researchers, who published their work in Science Immunology, created a blood test to pinpoint SARS-CoV-2 antibodies that target one unique piece of the SARS-CoV-2 spike protein. That piece is called a receptor binding domain, or RBD. Their RBD-based antibody test can measure the levels of that domain, which they found correlate to the levels of the all-important neutralizing antibodies that provide immunity.
The RBD of the spike protein in SARS-CoV-2 is not shared among other known human or animal coronaviruses. Therefore, antibodies against this domain are likely to be highly specific to SARS-CoV-2, and so these antibodies reveal if an individual has been exposed to the virus that can cause COVID-19. Indeed, when the researchers tested blood collected from people exposed to other coronaviruses, none had antibodies to the RBD of SARS-CoV-2.
“Our assay is extremely specific for antibodies to the virus that causes COVID-19, which is not the case for some currently available antibody tests,” said co-senior author Aravinda de Silva, professor of microbiology and immunology and member of the UNC Institute for Global Health and Infectious Diseases. “Our results strongly support the use of RBD-based antibody assays for population-level surveillance and as a correlate of the neutralizing antibody levels in people who have recovered from SARS-CoV-2 infections.”
First and co-senior author Prem Lakshmanane, Ph.D., assistant professor of microbiology and immunology at UNC, said, “We are now further streamlining our test into an inexpensive assay, so that instead of the test taking four to five hours to complete, our assay could be completed in about 70 minutes without compromising quality.”
During the UNC-Chapel Hill campus shutdown, Lakshmanane led a team of researchers including Ramesh Radi, Ph.D., Bruno Segovia-Chumbez, and Rajendra Raut, Ph.D. – each designated as an emergency employee—to develop the test from scratch. The team designed new antigens and used a large panel of SARS-CoV-2 patients and control human and animal samples. From day nine after the onset of symptoms and thereafter, the UNC assay allowed the researchers to accurately identify RBD-based antibodies to SARS-CoV-2.
World-renowned coronavirus expert Ralph Baric, Ph.D., Kenan Distinguished Professor of Epidemiology at the UNC Gillings School of Global Public Health, developed an assay to measure neutralizing antibodies in clinical samples. Assays for measuring neutralizing antibodies take about three days to complete and often require special high-containment facilities necessary for safely working with infectious viruses. The de Silva Lab collaborated with David Martinez, Ph.D., in the Baric laboratory to test if the RBD-based antibody levels in patients correlated with levels of neutralizing antibodies found in the Baric assay.
“We observed a robust correlation between levels of RBD-binding antibodies and SARS-CoV-2 neutralizing antibodies in individual samples,” Lakshmanane said. “This means our assay not only identifies people exposed to SARS-CoV-2, but it can also be used to predict levels of neutralizing antibodies and to identify potential donors for plasma therapy.”
The UNC-Chapel Hill researchers have received requests from scientists across the country and around the world for assistance with establishing this new assay within their research laboratories to monitor people for SARS-CoV-2 infection.
“We don’t see our research as a means to replace commercial tests,” said de Silva, a world-renowned arbovirus researcher. “Commercial tests are critical, especially for making decisions about the clinical management of individual patients. But it’s too early in the pandemic to know if the commercial assays are suitable for identifying people who experienced very mild or no disease after infection or if the assays tell us anything about protective immunity, as researchers are still learning about this virus.”
Source: Read Full Article