Pfizer Reports Their COVID-19 Vaccine Is 100% Effective in Kids Ages 12 to 15

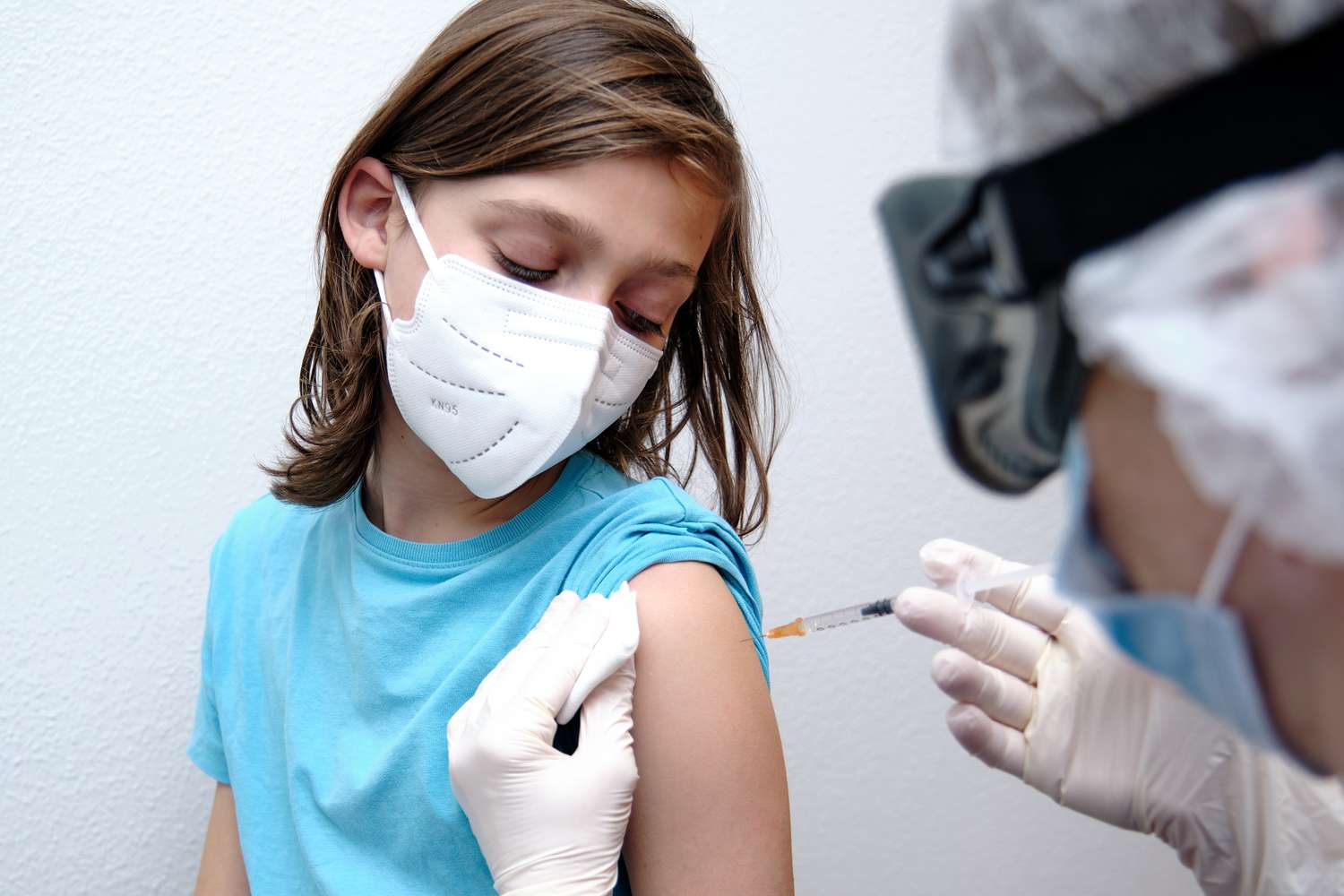
Children as young as 12 years old may soon be eligible for the Pfizer-BioNTech COVID-19 vaccine after the company said that it completely prevented COVID-19 illness in a clinical trial.
On Wednesday, Pfizer announced in a press release that their Phase 3 trials in adolescents aged 12 to 15 "demonstrated 100% efficacy and robust antibody responses," even "exceeding those recorded earlier in vaccinated participants aged 16 to 25 years old."
Half of the 2,260 study participants were placed in a placebo group and were given a saline solution, while the other half received the vaccine. In the placebo group, 18 participants contracted COVID-19 while none in the vaccinated group contracted the virus.
Pfizer added that the vaccine was also "well tolerated" by the 12-to-15-year-olds in the vaccinated group, who were a mix of kids who had never contracted COVID-19 and some who had.
Never miss a story — sign up for PEOPLE's free daily newsletter to stay up-to-date on the best of what PEOPLE has to offer, from juicy celebrity news to compelling human interest stories.
As with adults, the vaccine produced a "strong" immune response in children "one month after the second dose," Pfizer said.
Of the results, Pfizer Chairman and CEO Albert Bourla said in a statement that the company "share[s] the urgency to expand the authorization of our vaccine to use in younger populations and are encouraged by the clinical trial data from adolescents between the ages of 12 and 15."
Large Study Confirms COVID Vaccines Are Working in the U.S., Reducing Risk of Infection by 90%
"We plan to submit these data to FDA as a proposed amendment to our Emergency Use Authorization in the coming weeks and to other regulators around the world, with the hope of starting to vaccinate this age group before the start of the next school year," Bourla added.
Currently, Pfizer's vaccine is approved for use in people aged 16 and up, while the other two vaccines currently in use in the U.S., from Moderna and Johnson & Johnson, are approved for people 18 and older.
BioNTech Co-founder and CEO Ugu Sahin said in a statement, "Across the globe, we are longing for a normal life. This is especially true for our children. The initial results we have seen in the adolescent studies suggest that children are particularly well protected by vaccination."
"It is very important to enable them to get back to everyday school life and to meet friends and family while protecting them and their loved ones," Sahin added.
Pfizer and Moderna are also currently running clinical trials on their vaccine in children between the ages of 6 months and 12 years old.
Meanwhile, the COVID-19 vaccine rollout has picked up significantly in the U.S., with more than a dozen states now allowing anyone aged 16 and up to make an appointment.
As of March 31, nearly a third of the U.S. population, 96,044,046 people, have received at least one dose of a COVID-19 vaccine, the Centers for Disease Control says. Of that group, 16.1%, or 53,423,486 people, are fully vaccinated against the virus.
As information about the coronavirus pandemic rapidly changes, PEOPLE is committed to providing the most recent data in our coverage. Some of the information in this story may have changed after publication. For the latest on COVID-19, readers are encouraged to use online resources from CDC, WHO, and local public health departments. PEOPLE has partnered with GoFundMe to raise money for the COVID-19 Relief Fund, a GoFundMe.org fundraiser to support everything from frontline responders to families in need, as well as organizations helping communities. For more information or to donate, click here.
Source: Read Full Article