New research identifies injury-induced molecular changes in chondrocytes, at single-cell level

Articular cartilage is a connective tissue lining the surfaces of joints. When the cartilage severely wears down, it leads to osteoarthritis (OA), a debilitating disease that affects millions of people globally.
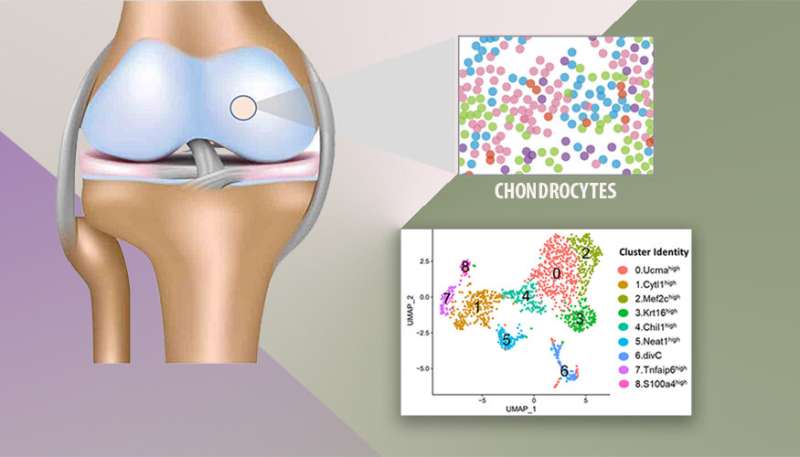
The articular cartilage is composed of a dense extracellular matrix (ECM) with a sparse distribution of cells found in healthy cartilage, also known as chondrocytes, with varying forms and potentially different functions. A deep, molecular level, understanding of various chondrocyte subpopulations and their functions is critical to develop effective methods for cartilage repair and regeneration.
Until now, a thorough molecular profiling of chondrocytes from healthy articular cartilage had not occurred due to technical difficulties in isolating and studying individual chondrocyte subpopulations from dense cartilage.
Lawrence Livermore National Laboratory (LLNL) scientists have profiled articular cartilage from healthy and injured mouse knee joints at a single-cell resolution and identified nine chondrocyte subtypes with distinct molecular profiles and injury-induced early molecular changes in these chondrocytes.
This is the first time scientists have identified all the chondrocyte subtypes in a healthy joint and conducted a 1:1 assessment of molecular changes associated with joint injury in these subpopulations of cells.
“This work expands our view of chondrocyte heterogeneity and rapid molecular changes in chondrocyte populations in response to joint trauma, and highlights potential mechanisms that trigger cartilage degeneration,” said LLNL computational biologist Aimy Sebastian, lead author of a paper appearing in Cells.
OA is a degenerative joint disorder that affects more than 300 million people worldwide, and often results in diminished quality of life and disability. Although OA prevalence is on the rise and the Center for Disease Control estimates that as many as 78 million Americans (1 in 4) will suffer from OA by 2040, an in-depth understanding of the joint microarchitecture and molecular mechanisms that contribute to OA initiation and progression remains in its early stages.
“The lack of sufficient progress in this area has severely hindered the development of effective therapeutic approaches for the early diagnosis, prevention and treatment of OA,” said LLNL biologist Gaby Loots, a co-author of the paper.
The team also compared mouse chondrocyte subpopulations to human chondrocytes and evaluated the extent of molecular similarity between mouse and human.
Source: Read Full Article