Nanoparticle therapy shows promise at preventing fatal newborn lung disease

A new therapy offers help for a disease so rare and complex that its acronym is hard to pronounce. For infants unlucky enough to be born with this lung disease, the outcome is usually fatal.
The disease is called ‘alveolar capillary dysplasia with misalignment of the pulmonary veins’ (ACDMPV). Research indicates the disease is linked to mutations in the FOXF1 gene. Worldwide, medical experts have documented about 200 cases, but an unknown number of infants may have died without the condition ever being diagnosed, according to the National Organization for Rare Disorders.
The disease is caused by genetic variations that prevent proper blood vessel formation in the lungs. Within days or weeks after birth, infants turn blue from lack of oxygen while blood pressure spikes within their lungs. The few who survive do so by receiving extremely rare infant-sized lung transplants.
Now, a study led by experts at Cincinnati Children’s and the University of Cincinnati reports helping mice (with a FOXF1 mutation identical to human ACDMPV patients) survive longer with this deadly disease by using high-tech nanoparticles to deliver a STAT3 gene into the lungs to stimulate blood vessel growth. STAT3 is a key downstream target of FOXF1, and its delivery can correct the vascular deficiency in ACDMPV mice. Details were published online June 11, 2021, in the journal Circulation.
If these results can be matched in human studies in the years to come, the co-authors say this success could boost the pace of development for other nanoparticle-based therapies for a wide range of conditions.
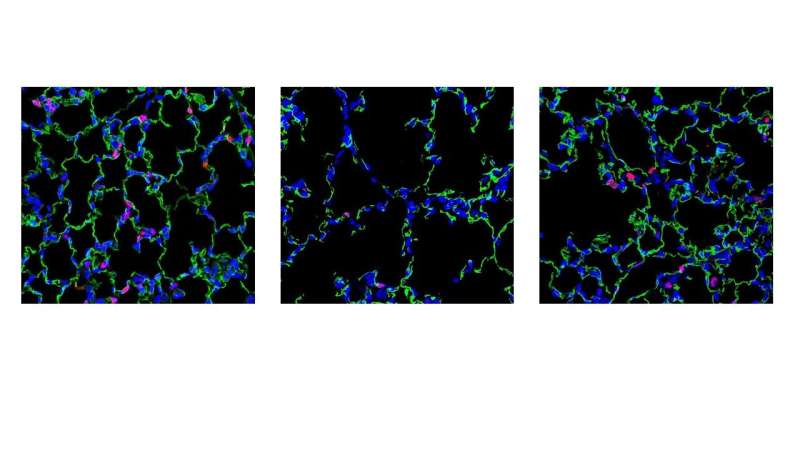
“Nanoparticle carriers have shown minimal toxicity and have accelerated the development of novel therapies for human cancers, diabetes and chronic inflammatory disorders. We have developed a unique nanoparticle delivery system that can deliver genes capable of stimulating micro-vessel growth in the newborn lung,” says the study’s senior author Vlad Kalinichenko, MD, Ph.D., a member of the Center for Lung Regenerative Medicine and the Perinatal Institute at Cincinnati Children’s. “This study shows that a single injection of the nanoparticles with the STAT3 gene vector was sufficient to increase alveolar capillary density, prevent excessively high blood pressures, and dramatically improve survival.”
Without treatment, about 70% of mice born with ACDMPV die within 28 days of birth. The new treatment reduced that mortality rate to 35%, says the study’s first author Fei Sun, Ph.D., a member of the Center for Lung Regenerative Medicine at Cincinnati Children’s.
Gene-driven therapy but not gene editing
Unlike gene replacement therapies that can make permanent changes to the body, this nanoparticle approach involves materials that do not stay in the body longer than seven days. And yet, in the mice studied, a single treatment early after birth was enough to divert an entire stream of later-developing problems that occur with ACDMPV.
The therapy works by delivering an engineered nanoparticle made of several polymers, fatty acids and a bit of cholesterol that carries the non-integrating STAT3 gene, which in turn prompts blood vessel growth in the lung tissue.
Kalinichenko and colleagues observed the molecular processes involved as part of their ongoing studies of lung development. The nanoparticle was developed with help from Zicheng Deng and Andrew Dunn, who are graduate students mentored by Donglu Shi, Ph.D., from the Materials Science and Engineering Program at the University of Cincinnati.
With more blood vessels in place, the rapidly growing newborn lungs formed in a closer-to-normal fashion, without setting off dangerous molecular “remodeling” signals that can cause permanent malformations and death from lung failure.
The study details how the treatment improved several measures of lung, heart and blood vessel health, including arterial oxygenation levels, blood pressure in the right ventricle, the ratio of pulmonary acceleration time to pulmonary ejection time (PAT/PET), the diameter of pulmonary arteries, and the thickness of their walls.
Next steps
Much more work must be completed before the nanoparticles can be tried in human newborns with ACDMPV, including safety tests and determining whether repeated treatments would be needed.
Source: Read Full Article