Macrophage to Foam Cell Differentiation Pathway

Macrophages differentiate into foam cells during the formation and progression of atherosclerosis. Understanding the process of this change during the progression of atherosclerotic plaques will allow for more efficient detection, treatments, and preventative measures for atherosclerosis.
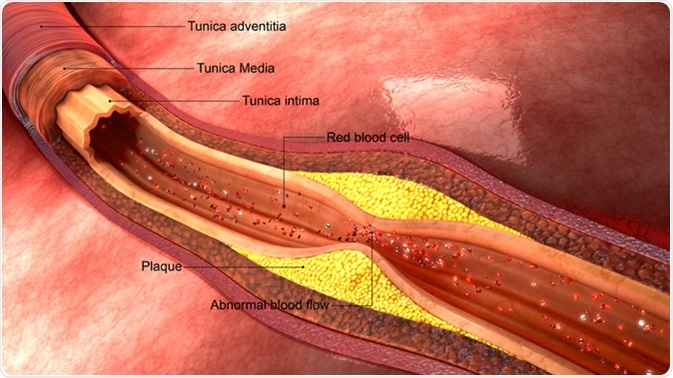
The Migration and Differentiation of Monocytes
Platelets have a key role in the regulation of inflammation at the site of an atherosclerotic plaque. Activated platelets release pro-inflammatory mediators, such as C-X-C motif chemokine ligand 12 (CXCL12). CXCL12 binds to receptors such as C-X-C motif chemokine receptor 4 (CXCR4) and CXCR7, which allows it to regulate cell migration, adhesion, and survival. The increased platelet-derived CXCL12 release is associated with a higher number of foam cells, as CXCL12 can induce the formation of macrophages which in turn can be differentiated into foam cells.
There is an increased amount of platelets at the site of vascular endothelial damage. The release of CXCL12 from the platelets causes migration of monocytes by binding to CXCR4. The binding of CXCL12 to CXCR7 causes the monocytes to adhere to the sub-endothelial layer, which is enriched in platelets.
The Role of Macrophages in Atherosclerosis
Macrophages have a critical role in the formation of atherosclerosis. When the endothelial wall within a blood vessel is damaged, monocytes are recruited into the sub-endothelial space and start to differentiate into M1 macrophages. These macrophages also release pro-inflammatory cytokines, such as macrophage colony-stimulating factor (M-CSF), which induce the differentiation of more monocytes into M1 macrophages. The macrophages also release chemokines, such as C-C chemokine receptor 7 (CCR7), which results in the migration of more monocytes to the site of the plaque.
This increases the number of M1 macrophages present, which allows an increased response against the atherosclerotic fatty streaks. This response triggers the recruitment of other immune factors and phagocytosis of platelets and low-density lipoprotein cholesterol (LDL-C). Anti-inflammatory M2 macrophages are also present in advanced atherosclerotic lesions.
This type of macrophage is induced by interleukin (IL)-4 or IL-10. M2 macrophages have increased IL-10 and suppressed IL-12 secretion, which reduces excessive inflammation and facilitates collagen production and fibrosis, which aids with healing. Macrophages can change their phenotype back and forth from M1 to M2. Previous studies have shown that the increase in M1 to M2 differentiation is correlated to the regression of plaques in atherosclerotic mouse models.
Macrophage to Foam Cell Transition
The induction of foam cell formation has several causes. The most prominent being the death of macrophages resulting from excessive phagocytosis of low-density lipoprotein (LDL). The stimulation of macrophages by certain ligands, as well as pathogen-induced signaling also contribute to the formation of foam cells from macrophages.
Phagocytosis of Low-Density Lipoproteins
The transition from macrophages to foam cells is a key step that occurs when fatty streaks are formed during the development of atherosclerotic plaques. The formation of foam cells occurs when macrophages engulf an excess of many types of LDL, including oxidized LDL (OxLDL) and minimally modified LDL (mmLDL), which result in the death of the macrophages once they become oversaturated with LDLs. This occurs at fatty streaks, which is the early stage of atherosclerotic plaque development. The accumulation of foam cells contributes to the development of atherosclerosis by progressing plaque formation and causing the formation of unstable plaques.
Lipid Signaling
Lipid polysaccharide (LPS) signals through toll-like receptor 4 (TLR4) to activate the immune response via the activation of NF-kB, AP-1 or IFN production. OxLDL signals through TLR4, as well as CD36 and TLR6, which impairs the LPS-induce immune response and induces the formation of foam cells. This signaling changes the metabolic state of macrophages resulting in decreased cholesterol efflux, which causes increased foam cell formation.
Pathogen-Induced Signaling
Pathogens can induce the uptake of LDL by macrophages leading to the formation of foam cells. Porphyromonas gingivalis and Chlamydia pneumoniae are two pathogens that are believed to be associated with the development of atherosclerosis. Research using bone marrow-derived macrophages (BMDM) discovered that both P. gingivalis and C. pneumoniae induce the formation of foam cells. Infection with both of these pathogens causes the secretion of cytokines, such as TNF-α and IL-6, while C. pneumoniae infection also causes IL-1β secretion. These changes lead to a proinflammatory response in macrophages, which contribute to the formation of foam cells.
Complications of Atherosclerosis
As discussed, the formation of foam cells leads to the progression of fatty streaks that eventually turn into very developed plaques. This change leads to atherosclerosis in the arteries of an individual. The plaques can narrow arteries and even lead to the formation of blood clots. Because of this, atherosclerosis often develops into diseases, such as coronary heart disease and strokes.
Elucidation of the mechanisms of macrophage to foam cell differentiation will help to steer us in the right direction with regards to managing atherosclerosis.
Sources
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3320912/
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4670914/
- http://www.eurekaselect.com/60199/article
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3478499/
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5985511/
Further Reading
- All Macrophage Content
- What is a Macrophage?
- Macrophage Function
- What is the difference Between a Phagocyte, Macrophage, Neutrophil and Eosinophil?
Last Updated: Oct 29, 2018
Written by
Samuel Mckenzie
Sam graduated from the University of Manchester with a B.Sc. (Hons) in Biomedical Sciences. He has experience in a wide range of life science topics, including; Biochemistry, Molecular Biology, Anatomy and Physiology, Developmental Biology, Cell Biology, Immunology, Neurology and Genetics.
Source: Read Full Article