How do immunocompromised patients respond to COVID-19 vaccines?

The COVID-19 vaccines produce a boost in antibody activity against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). However, new research suggests vaccine responses aren’t all created equal. Their findings showed that immunocompromised patients, such as transplant recipients, with no prior exposure to SARS-CoV-2 produce little to no neutralizing antibodies.
The researchers write:
“Consistent with findings of other investigators, we show that COVID-19-naive heart and lung transplant recipients have a profound reduction in neutralizing antibodies against SARS-CoV-2 spike protein and receptor binding domain-specific immunoglobulin levels after both prime and boost vaccine doses. However, transplant recipients who had COVID-19 infection prior to vaccination displayed neutralizing antibodies to SARS CoV-2 at baseline that increased following vaccination.”
The study “Vaccine serologic responses among transplant patients associate with COVID-19 infection and T peripheral helper cells” is published and available on the medRxiv* preprint server.
.jpg)
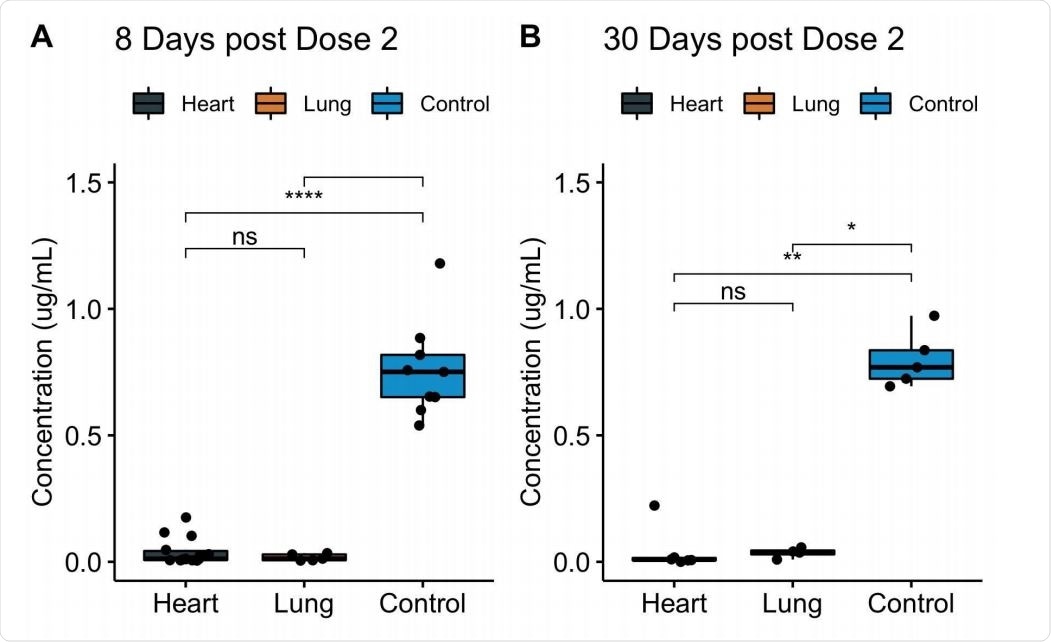
Transplant recipients had lower antibody levels from vaccination
The researchers measured vaccine-induced antibody responses in patients who received heart and lung transplants. They compared the effectiveness of these patients’ antibody levels against several variants of concerns. Lastly, they profiled the patients’ adaptive immune response, including CD4+ 74 T helper cells, CD8+ T cells, and B cells.
Vaccinated heart and lung transplant recipients with no history of COVID-19 infection had lower IgG levels that would recognize and target the SARS-CoV-2 receptor binding domain than non-immunocompromised people.
The antibody levels in vaccinated transplant patients were 22-fold lower after eight days of receiving the second COVID-19 vaccine. After 30 days of the second dose, IgG levels were 20-fold lower than people considered healthy and vaccinated.
Decreased neutralizing activity against SARS-CoV-2
Similar to their IgG levels, transplant recipients also showed diminished neutralizing antibody activity against SARS-CoV-2.
Eight days after receiving the second vaccine, transplant recipients had 12.2-fold less neutralizing activity than healthy controls. Thirty days after the second dose, neutralization was 12-fold less than healthy controls.
Transplant recipients also had lower neutralization against variants of concern with E484K/N501Y/D614G mutations such as the beta (B.1.351) and gamma (P.1) variants.
Whether it was before or weeks after the second dose, neutralization against these variants was 22% lower than neutralizing the D614G variant. Against a virus similar to the Alpha variant (B.1.1.7) with the N501Y/D614G mutation, there was a 24% neutralization. However, the researchers note that neutralization differences between D614G and N501Y/D614G were nonsignificant.
Both transplant recipients and healthy controls strongly correlated IgG antibody levels targeting SARS-CoV-2’s receptor-binding domain and neutralizing antibody titers. However, this correlation was reduced when mutations were added to the mix.
Antibody response did increase over time in transplant recipients
Transplant recipients took longer than healthy controls to show increases in IgG antibody levels 20 days after their first vaccine dose. After the second vaccine dose, IgG levels and neutralizing activity gradually rose in two transplant recipients and the rest of the healthy controls. Increased neutralizing activity was associated with an increase in IgG levels. Although, this simultaneous increase in IgG levels took longer with transplant recipients than healthy controls.
Three transplant recipients had previously recovered from COVID-19 infection before getting vaccinated. However, one had become infected between their first and second vaccine dose.
Of the 4 transplant recipients, 3 showed comparable IgG levels and antibody neutralizing activity to healthy controls. Once vaccinated, there was a boost in both IgG levels and neutralizing activity.
Overall, transplant recipients who showed a vaccine response differed from vaccinated transplant recipients with prior COVID-19 infection.
“Proximity to transplantation and the higher doses of immunosuppressive medications generally administered around transplantation may have contributed to the absence of response in the COVID-19-infected non-responder, as this individual developed infection <1 year after transplantation, whereas the other three COVID-19-infected transplant recipients developed infection 2-5 years and >15 years after transplantation,” explained the researchers.
Immune response
Transplant recipients showed significant differences between CD3+ lymphocytes, CD4+ T lymphocytes, T peripheral helper cells, Tregs, and DR+ T cells.
There were reduced TPH/CD4+ and DR+/CD4+ cells in transplant patients than in healthy controls.
T peripheral helper cells had the strongest correlation towards antibody response.
“Taken together, the disparate humoral immune response to SARS-CoV-2 vaccine as compared to natural infection in heart and lung transplant patients is suggestive of a failure of vaccines to induce immunologic priming in highly immunosuppressed patients, and points to the need for alternate immunization regimens for these populations,” concluded the researchers.
*Important Notice
medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
- Lemieux JL, et al. Vaccine serologic responses among transplant patients associate with COVID-19 infection and T peripheral helper cells. medRxiv, 2021. doi: https://doi.org/10.1101/2021.07.11.21260338, https://www.medrxiv.org/content/10.1101/2021.07.11.21260338v1
Posted in: Men's Health News | Medical Research News | Medical Condition News | Women's Health News | Disease/Infection News
Tags: Antibodies, Antibody, CD3, CD4, Coronavirus, Coronavirus Disease COVID-19, Heart, Immune Response, Immunization, Immunoglobulin, Lung Transplant, Mutation, Protein, Receptor, Research, Respiratory, SARS, SARS-CoV-2, Severe Acute Respiratory, Severe Acute Respiratory Syndrome, Spike Protein, Syndrome, Transplant, Vaccine, Virus

Written by
Jocelyn Solis-Moreira
Jocelyn Solis-Moreira graduated with a Bachelor's in Integrative Neuroscience, where she then pursued graduate research looking at the long-term effects of adolescent binge drinking on the brain's neurochemistry in adulthood.
Source: Read Full Article